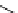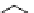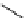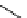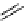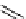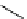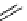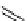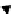Chemistry Reference
In-Depth Information
NCS
*
P(O)Cl
3
NH
2
S
P
2
S
5
380 °C
S
-
H
11
CS
2
> 95%
*
11
CH
3
I
CH
3
I
S
H
S
*
scheme 4.14
Preparation of
11
Cs
2
from
11
CH
3
i and subsequent reactions with benzylamine to form a C-11 dithiocarbamate, C-11
dithiocarbamate ester and an C-11 isothiocyanate. [*] indicates labelling position.
AgNO
2
11
CH
3
I
(g)
11
CH
3
NO
2
100 °C
scheme 4.15
Conversion of
11
CH
3
i to
11
CH
3
NO
2
via reaction with AgNO
2
.
OH
NO
2
R
nitroaldol reaction
*
NH
2
NH
2
O
O
*
HO
OH
*
H
RH
OH
1.
[C-11]Tris
R = phenyl,
ethylphenyl
2. LiAlH
4
1. formaldehdye,
TBAF, THF
2. NiCl
2
/ NaBH
4
O
11
CH
3
NO
2
1.
OH
OH
1. TBAF, THF
OH
O
D-arabinose
OH
Ph
OCH
3
O
N
2. Nef
reaction
2. NiCl
2
/ NaBH
4
1.
N
N
2. Zn,
HCl/EtOH
EtO
OH
OH
H
2
N
*
O
O
O
*
*
OH
OH
Ph
OCH
3
OH
OH
OH
OH
+
OH
O
Michael addition
NH
2
OH
EtO
*
[C-11]D-mannose
[C-11]D-glucose
C-carboxylation
scheme 4.16
C-11 radiolabelling reactions using
11
CH
3
NO
2
.
outlet has been found to improve the quality of
11
CH
3
NO
2
by removing nitrogen oxide compounds that are the result of
AgNO
2
pyrolysis [68].
Although
11
CH
3
NO
2
is a much less reactive molecule than the other
11
CH
3
i-derived precursors described above, it has
been applied to the synthesis of a number of labelled compounds (scheme 4.16). it is a versatile reagent that has not been
widely exploited in the field. The condensation reaction of
11
CH
3
NO
2
with D-arabinose has been used to form the epimeric
mixtures of [C-11]D-nitroalcohols, which can then be subsequently converted to [C-11]D-glucose and [C-11]D-mannose
via the Nef reaction (oxidation of nitro compounds to carbonyls). [C-11]D-mannose was formed as the major product; both