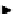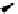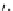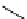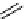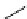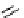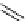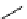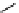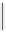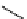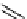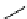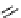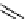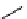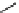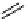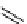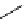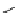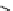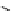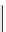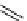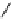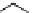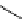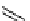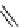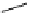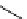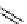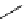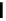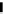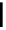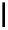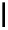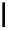Chemistry Reference
In-Depth Information
Br
11
CH
3
F
N
11
CH
3
I
TBAF
(TMS)
3
N
11
CH
3
Sn[N(TMS)
2
]
2
Sn
Pd Cat.
(TMS)
3
N
F
N
scheme 4.11
synthesis of [
11
C]monomethylstannate (
11
CH
3
snF
2
[N(TMs)
2
]
2
) and reaction with 3-bromoquinoline to give
[
11
C]3-methylquinoline.
X
N
Sn
11
CH
3
nBuLi
R
Cl
N
11
CH
3
I
11
CH
3
Li
Sn
Pd Cat.
R
11
CH
3
scheme 4.12
Preparation of the [
11
C]methyl transfer reagent 5-[
11
C]methyl-1-aza-5-stanna-bicyclo[3.3.3]undecane and stille cross-
coupling reaction with aryl or vinyl halides.
NH
2
H
NH
*
Me
3
+
+-
11
CH
3
I, DM
F
11
CH
2
O
O
-
Me
3
NO
O
H
p-TsOH, DMF, 70°C
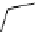
scheme 4.13
Preparation of
11
CH
2
O from
11
CH
3
i and subsequent reaction with tryptamine to form [
11
C]-2,3,4,9-tetrahydro-1H-b-
carboline. Ts = toluenesulfonyl. [*] indicates labelling position.
undecane. Coupling reactions of aryl and vinyl halides were found to give best rCy when carried out in the presence of a palla-
dium allyl catalyst at temperatures above 100 °C. However, rCys are highly dependent on the arylhalide substrate. One drawback
of this system is the difficulty associated with the preparation of
11
CH
3
Li which may be responsible for inconsistent rCys.
Although methyl iodide has proven to be an exceptionally important labelling precursor, it is limited to labelling on the
periphery of target molecules. For the majority of precursor compounds, a reactive nucleophilic functional group, such as an
amine or alcohol, is required in the structure. in order to label a wider variety of atomic positions and functional groups
within the skeletal or ring structure of a molecule, more diverse and reactive secondary precursor molecules are needed.
Although is C-11 methyl iodide is a secondary precursor, its production is now so routine, fast, and reliable at many PET
centres that it can in fact be used as starting point to form other reactive secondary labelling precursors. Hooker et al. recently
reported a convenient synthesis of C-11 formaldehyde from C-11 methyl iodide [64]. Conventionally, C-11 formaldehyde is
produced either by the partial reduction of
11
CO
2
[65] or by the oxidation of C-11 methanol; [66] both routes result in a mix-
ture of C-11 labelled products. By using trimethylamine N-oxide (TMAO) as an oxidant, it was possible to achieve instan-
taneous conversion of
11
CH
3
i to
11
CH
2
O in high radiochemical yields (>80%) (scheme 4.13), thus providing a particularly
convenient and high yielding route that should be easy to adopt by other PET centres. Furthermore, Hooker et al. were able
to demonstrate that
11
CH
2
O could be used to label [
11
C]-2,3,4,9-tetrahydro-1H-b-carboline in the ring structure.
The rapid gas phase conversion of methyl iodide to the versatile electrophilic reagent C-11 carbon disulfide has been
recently reported by Miller et al. [67] Despite carbon disulfide being a widely known reagent in the chemical industry its
C-11 radiochemistry had not been previously investigated up to this point. it was reported that
11
CH
3
i could be readily con-
verted to
11
Cs
2
in high rCys within a short reaction time (<10 min., from end of
11
CH
3
i production) via a high temperature
(400 °C) gas phase reaction with P
2
s
5
.
11
Cs
2
could be conveniently trapped in acetonitrile at room temperature, making it
easy to handle and process. reaction with primary, secondary, and aromatic amines proceeded very efficiently to form C-11
dithiocarbamates (scheme 4.14) in quantitative yields and within 5 min. in reaction times. subsequent reaction of the C-11
dithiocarbamates with alkylhalides afforded quantitative conversion to the dithiocarbamate esters, while reaction of the
benzyl dithiocarbamate with POCl
3
resulted in desulfurisation and formation of the corresponding isothiocyanate.
C-11 nitromethane is another reagent that can be quickly generated from
11
CH
3
i. it is typically prepared via passing a gas
stream of
11
CH
3
i through a column of AgNO
2
that is heated to 100 °C. The conversion is fast, complete in a few minutes, and
produces
11
CH
3
NO
2
with conversions of about 90% (scheme 4.15). An additional column of NaHCO
3
attached to the AgNO
2