Biomedical Engineering Reference
In-Depth Information
A.
Bone Ingrowth
B.
Bone Surface Area
C.
Bone Fill
50
1.75
100
1.50
40
80
1.25
Ti
30
1.00
60
Ti-CaP
0.75
20
Ti-TGF-
β
1
40
0.50
20
10
0.25
0
0.00
0
= p < 0.0005
= p < 0.0005
= p < 0.0005
= p < 0.0005
= p < 0.01
= p < 0.005
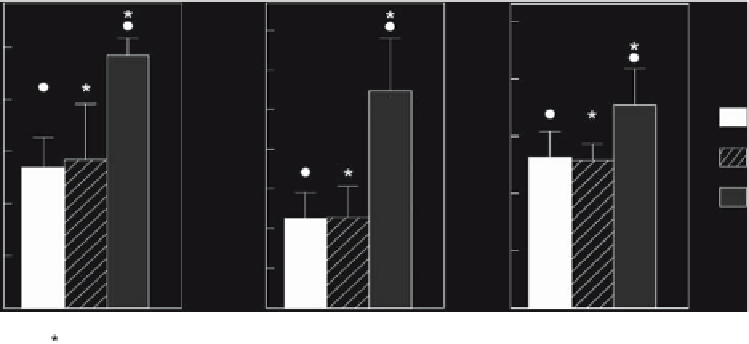
Figure 5.5.
(A) Bone ingrowth in various titanium implants, (B) bone surface area, and (C) bone fill. The results of the paired
t
-test comparing titanium (Ti) with titanium-calcium phosphate (Ti-CaP) and of the
t
-tests comparing titanium with Ti-TGF-
β
1
and
Ti-CaP with Ti-TGF-
β
1
are indicated. Significant differences between Ti and Ti-TGF-
β
1
(•) and between Ti-CaP and Ti-TGF-
β
1
(*) are
indicated. No significant difference was found between Ti and Ti-CaP implants for any parameter (
p
>
0.05).
model. Calcium phosphate-coated and -non-
coated porous titanium implants, half of them
loaded with rhTGF-
β
1
from the
titanium fi ber meshes showed a burst of release
during the fi rst
A study of in vitro release of TGF-
β
1
, were bilaterally im-
planted and left to ingrow. Histological analy-
sis demonstrated that in the TGF-
%
release had occurred. Following the burst, a
slower phase liberated
2
hours, when more than
70
β
1
-loaded
implants, bone had formed throughout the im-
plant up to the center, whereas in the absence
of growth factor, only partial ingrowth of bone
was observed. The bone had a trabecular
appearance and was present along with bone
marrow-like tissue. All histological fi ndings
were confi rmed by image analysis:
80
% of the theoretical
dose by
week. It thus seems that a dose-
response relationship exists for TGF-
1
β
1
release
with respect to bone induction. Higher doses
do not necessarily generate more bone forma-
tion; rather, there is an optimum dose [
].
Taken together, these results show that the
combination of titanium-mesh with TGF-
3
,
4
97
%
β
1
ingrowth was seen in the rhTGF-
β
1
-loaded
can induce orthotopic bone formation [
40
].
implants, whereas only
% ingrowth
was observed in the nonloaded calcium phos-
phate-coated and -noncoated implants, respec-
tively. Bone surface area and bone fi ll were
signifi cantly higher in the rhTGF-
57
% and
54
5.7 Conclusions
β
1
-loaded
mm
2
and
implants (
%, respectively) than
in the nonloaded implants (
1
.
37
36
Autologous bone or bone derivatives and sub-
stitutes for bone reconstruction have signifi -
cant limitations in terms of availability,
morbidity, effi cacy, immunologic reaction, and
disease transmission. As a result, novel tissue-
engineering models have been designed to
overcome these problems. The factors neces-
sary for tissue engineering include cells, the
scaffold for cell proliferation and differentia-
tion, and growth factors. For example, one
practical way to provide an environment suit-
able for induction of tissue regeneration at a
defect involves placing a scaffold as an artifi cial
mm
2
and
0
.
57
26
%)
(Fig.
). There were no statistically signifi cant
differences in any parameter between the
calcium phosphate-coated and -noncoated
implants. Quadruple fl uorochrome labeling
showed that in the titanium and titanium-
calcium phosphate implants, bone guidance
had occurred from the former defect edge,
whereas in the titanium-TGF-
5
.
5
β
1
implants, bone
formation had been initiated in the center of
the pore and proceeded in a centrifugal
manner.