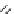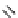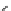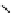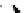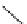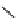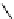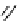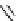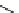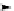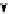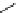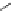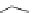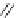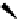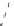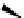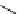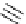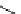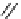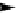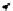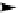Chemistry Reference
In-Depth Information
CH
2
Cl
2
, 25 ˚C
then
MeCN, reflux
Me
Me
N
N
Ar
Ar
N
N
Ar
Ar
Pd
MeHN
NHMe
N
N
Cl
Cl
C
C
Pd
65%
Cl
Cl
Ar = 4-CF
3
-C
6
H
4
air, DMSO
or
PhI=O, DMF
Cl
Cl
Pd
Ar
N
NAr
Ar
N
N
Ar
MeNC
MeN
MeN
NMe
NMe
DMF
67%
69%
Scheme 3.22
Synthesis of bicyclic bisamidine fromdiamine and palladium isonitrile complex
CO
2
R
3
R
1
R
1
a
: R
1
= H, R
2
= Ph, R
3
= Me (82%)
b
: R
1
, R
2
=-(CH
2
CH
2
CH=CHCH
2
CH
2
), R
3
= Et (67%)
c
: R
1
, R
2
= (CH
2
)
4
, R
3
= Et (45%)
BF
3
·OEt
2
N
CO
2
R
3
N
Me
R
2
MeCN
N
R
2
Scheme 3.23
Synthesis of cyclic amidines from aziridines and acetonitrile
H
3
CO
2
C
BF
3
MeO
2
C
BF
3
N
COMe
N
COMe
Me
N
MeCN
F
3
B
COMe
N
MeO
2
C
MeO
2
C
COMe
N
Me
N
N
Me
Scheme 3.24
Chiral cyclic amidine from N-acylaziridine-2-carboxylate and acetonitrile