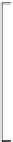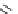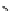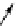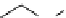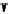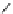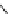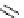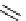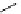Chemistry Reference
In-Depth Information
Ph
Ph
HN
Ar
Ar
N
H
Ar =
p
-
t
Bu-C
6
H
4
H
2
N
NH
HCl
1. BuLi
2. Cp
2
ZrMe(OTf)
THF
Et
2
O
HCl
91%
Ph
Ph
N
Ar
TMS-N=C=N-TMS
Cp
2
Zr
N
O
TMS
Cp
2
Zr
Ph
Ar
N
N
TMS
N
N
Cp
2
Zr
C
TMS
N
Ar
TMS
11
Scheme 3.25
Insertion of carbodiimides to zirconaaziridines for
the preparation of
aminoamidines
aziridine ring is opened by nucleophilic attack of nitrile at the less-hindered position 3 and
the formed ynimium is recyclized. As a Lewis acid catalyst, trimethyloxonium tetrafluor-
oborate (Meerwein reagent) (Me
3
O
þ
BF
4
) [41], scandium triflate [Sc(OTf)
3
] [42] and
cupric triflate [Cu(OTf)
2
] [43] are also effective, among which Cu(OTf)
2
could be
recommended because of easy handling and wide applicability to aziridine substrates.
3.2.8.2
Zirconaaziridine
Carbodiimides are potential nitrogen sources for amidines [44]. Zirconaaziridines, gener-
ated in situ from amines, butyllithium (BuLi) and bis(
5
-cylopentadienyl)methyl
(trifluoromethanesulfonyl)zirconium [Cp
2
ZrMe(OTf)], are efficiently trapped by carbo-
diimides. Zirconacycles 11, produced by insertion of carbodiimides into the Zr-C
bond of zirconaaziridines, are supposed to be key intermediates, which are hydrolyzed
to give
Z
a
-aminoamidines (Scheme 3.25).
3.3 Application of Amidines to Organic Synthesis
3.3.1 Acetoxybromination
Isoamarine (8), a cyclic amidine, is used for the transfer of electrophilicbromine fromNBS to
vinylarenes [45]. Thus, styrene is acetoxybrominated with NBS in the presence of catalytic
isoamarine(0.01equiv.) inaceticacid(AcOH) toaffordabromoacetate in95%yield.Asingle
anti-diastereoisomer is obtained when the 2-substituted derivative is used (Scheme 3.26).
The catalytic cycle shown in Scheme 3.27 is proposed, in which isoamarine acts as an
electrophilic bromine carrier from NBS. A related brominated 2-phenylamidine, which is