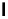Chemistry Reference
In-Depth Information
Mn(TPP)X
(5-10 mol%)
PhI=NTs
[ X(TPP)Mn=NTs ]
[2+2] cycloaddition
MeCN
X = ClO
4
Ts
NTs
N
X(TPP)Mn
N
63%
X(TPP)Mn
Me
Me
N
Ts
N
N
H
Me
Scheme 3.21
Oxidative amidination of cyclohexene with PhI
¼
NTs and acetonitrile
catalysed by Mn(TPP)ClO
4
aziridination, and a stoichiometric amount of (N-tosylimino)phenyliodinane (PhI
NTs)
in acetonitrile in the presence of a catalytic amount of manganese (III) tetraphenylporphyrin
perchlorate [Mn(TPP)ClO
4
] gives N-tosylamidine. In this reaction a metal-imido inter-
mediate is initially formed from Mn(TPP)ClO
4
and PhI
¼
¼
NTs, and then three sequential
reactions - [2
2] cycloaddition with acetonitrile (MeCN) [37], rearrangement to imino-
nitrenide and insertion at the allylic position of cyclohexene - occur (Scheme 3.21). Use of
Mn(TPP)ClO
4
gives the desired N-tosylamidine in 63% yield, whereas the corresponding
chloride [Mn(TPP)Cl] gives a lower yield (27%).
þ
3.2.7 Oxidative Cyclization to Bisamidine
Bicyclic bisamidines can be prepared from trans-N,N
0
-dimethyl-1,2-diaminocyclohexane
and a palladium bis(arylisocyanide) complex [38] (Scheme 3.22). Oxidation of the
initially-formed palladium bis(acyclic diaminocarbene) complex with air or idosobenzene
(PhI
O) followed by treatment with excess amounts of methylisonitrile (MeNC) yields
bicyclic bisamidines. The structure of the product obtained in each step is unequivocally
determined by X-ray crystallographic analysis.
¼
3.2.8 Ring Opening of Aziridine
3.2.8.1 Aziridine
Lewis acid catalysed [3
2] cycloaddition of N-alkoxycarbonylaziridines and cyanoalkane
such as MeCN furnishes 2-imidazolidines [39] (Scheme 3.23). Although ring-fused
aziridines are useable as substrates, cis-stereochemistry in the ring juncture is isomerized
to trans-stereochemistry in the bicyclic amidine products. Benzonitrile also serves as a
nitrile source.
Application to chiral N-acylaziridine-2-carboxylate gives the corresponding chiral 2-
imidazolidine with the retention of configuration [40] (Scheme 3.24). This implies that the
þ