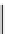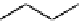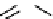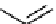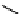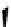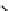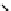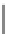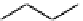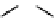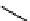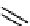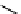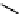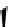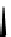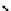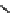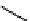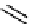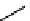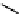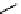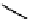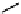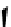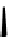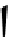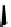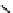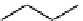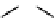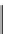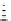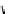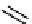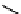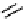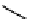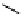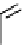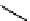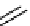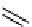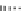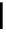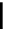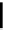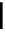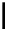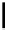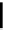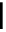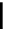Chemistry Reference
In-Depth Information
R
Me
Me
H
N
H
N
N
N
N
N
N
N
N
N
H
N
H
Me
Me
Me
glomerulatine A (
16
): R = Me
glomerulatine B (
17
): R = H
glomerulatine C (
18
)
(-)-calycanthine
Me
Me
H
N
N
N
N
N
N
H
N
H
Me
Me
isocalycanthine
19
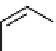
Figure 10.5
Structures of glomerulatines A-C (16-18) and related compounds
comparison of
1
H-NMR data with the computer analysis data (Figure 10.4). Total synthesis
of racemic perophoramide has been reported [36b].
10.2.3 Natural Amidines from Higher Plants
10.2.3.1 Glomerulatines
Glomerulatines A-C (16-18) were isolated from the aerial part of a shrub, Psychotria
glomerulata (Don. Smith) Steyermark, previously known as Cephaelis glomerulata J.D.
Sm [37]. The dimeric structures were determined by spectroscopic means. The absolute
configuration of glomerulatine A (16) was deduced from those of (
)-calycanthine, which
possesses similar optical rotation ([
)-calycanthine). On the
other hand, isocalycanthine type amidine compound, (8-8a),(8
0
-8
0
a)-tetrahydroisocaly-
canthine (19) was isolated from Psychotria colorata (Willd. ex R. and S.) Muell. Arg.,
which belong to the same genus as P. glomerulata [38] (Figure 10.5).
a
]
D
466 for 16,
489 for (
10.3 Natural Guanidine Derivatives
In this section, natural products with guanidines are studied. A large group of these products
consist of cyclic depsipeptides and polypeptides with arginine as an amino acid residue,
produced by mainly actinomycete and cyanobacteria in some cases (ex. microcystin-LR,
noduralin) [39]. Isolation of aminoglycosides (streptomycins, streptothricins [40] and their