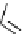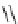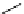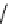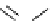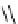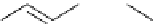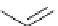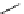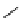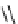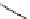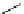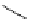Chemistry Reference
In-Depth Information
HBTU (1 equiv)
PS-BEMP
(3 equiv)
NOH
O
N
+
RCOOH
H
2
N
Ar
R
Ar
MW, MeCN
160 °C, 15 min
N
O
N
O
N
NO
2
CF
3
N
N
BnO
BnO
89%
81%
O
N
N
Me
87%
Scheme 6.17 Preparation of 1,2,4-oxadiazoles via condensation of amidoximes with
carboxylic acids under the irradiation of microwave
[69]. On the other hand, 1,2,4-oxadiazole derivatives could be prepared efficiently by the
condensation of amidoximes with carboxylic acids in the presence of one equivalent of
O-(benzotriazol-1-yl)-N,N,N
0
,N
0
-tetramethyluronium hexafluorophosphate (HBTU) and
three equivalents of PS-BEMP under microwave irradiation [70] (Scheme 6.17). This
method is highly desirable in an automated format where most of the reagents can be added
through liquid-delivery lines, and the PS-BEMP reagent can be easily removed by filtration
upon completion of the reaction.
For the rapid on-demand synthesis of 4,5-disubstituted oxazole libraries, a flow reaction
system equipped with two columns containing PS-BEMP and QP-BZA (QuadraPure
benzylamine, a primary amine functionalized resin), respectively, was developed by Ley
et al. [71,72] (Scheme 6.18). This system is very valuable from the viewpoint of rapid access
to 25 different oxazoles in yields between 83 and 99%, the easewith which the reactions can
be scaled-up to 10 g and the ability to recycle the PS-BEMP containing columns between
runs.
6.5 Miscellaneous
In the molecular engineering of carbohydrates, glycosyl trichloroacetimidates play an
important role as effective glycosyl donor molecules; they are generally prepared by
trapping anomeric alcohols by treatment with trichloroacetonitrile in the presence of a
strong base [73]. However, purification is sometimes diminished by the acid lability of
trichloroacetimidate functionalities. To reduce such an inconvenience to remove trace