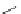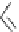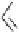Chemistry Reference
In-Depth Information
flow reactor
RCOCl
R
O
+
in MeCN
PS-BEMP
QP-BZA
EWG
N
N
EWG
C
QP-BZA
=
primary amine-functionalized resin
Br
Me
N
l
Me
N
O
N
O
N
Me
O
O
N
O
O
O
N
N
N
N
N
COOEt
COOEt
COOEt
COOEt
COOEt
88%
93%
89%
99%
92%
Scheme 6.18
Automated flow synthesis of 4,5-disubstituted oxazoles
amounts of base, polymer-supported superbase reagents such as PS-TBD, PS-DBU and
PS-BEMP have been developed. Clearly, this constitutes one of the most powerful
glycosylation methods in synthetic glycochemistry [74-76] (Scheme 6.19). In addition,
an advantage of the method is that the polymer-supported reagents can be regenerated and
reused.
Dondoni et al. further extended this chemistry to the highly sophisticated synthesis of
trisaccharides by iterative glycosidation using a standard trichloroacetyl isocyanate-based
sequestering technique [77] (Scheme 6.NaN). In this sequence, PS-BEMP played a key role
in removing impurities arising from excess unreacted glycosyl acceptors in its trichlor-
oacetyl urethane form, to provide a chromatography-free purification technique for
obtaining a variety of oligosaccharides with high purities.
The PS-TBD catalyst has been shown to be effective for epoxide ring opening reactions
with several nucleophiles such as thiols under solvent free conditions [37,78] (Scheme
6.21). In this case, the reusability of the catalyst was also established without a significant
loss of reactivity and selectivity. As a related work, the utility of mesoporous silica-
supported TBD catalysts was demonstrated in the reaction of propylene oxide with carbon
dioxide to prepare the corresponding carbonate derivative under the ultrasonic activation
[79].
PS-TBD
or
PS-DBU
OBn
OBn
O
O
BnO
BnO
BnO
BnO
+ Cl
3
CCN
O
CCl
3
OH
or
PS-BEMP
CH
2
Cl
2
, rt
(5 equiv)
OBn
OBn
NH
>99% yield
(
α / β
= 1 : 1 - 3 : 2)
Scheme 6.19
Preparation of glycosyl trichloroacetimidates