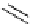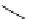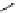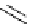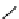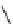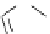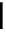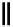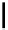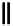Chemistry Reference
In-Depth Information
NH
2
O
N
R
1
PS-TBD
S
S
+
47-95% yield
Br
H
2
N
NH
2
THF
reflux, 0.5 h
R
1
R
3
R
3
R
2
R
2
Scheme 6.15
Preparation of 2-amino-4-aryl-1,3-thiazoles via condensation of
a
-bromo-
acetophenones with thiourea
(Viagra) was built up by the PS-BEMP promoted intramolecular alkylation of nitriles
[49].
Graybill et al. discovered a new expeditious method for preparing a variety of 3-thio-
1,2,4-triazole derivatives starting from acyl hydrazides and isothiocyanates via PS-BEMP
mediated multi-step transformations: the so-called
methodology
[65] (Scheme 6.16). In this sequence, PS-BEMP was successfully used for both the
deprotonation of acyl hydrazides and N-alkylation of weakly acidic triazoles. Using the
conceptually analogous cyclodehydration strategy, a variety of 2-amino-1,3,4-oxadiazole
derivatives, an important class of heterocycles in medicinal chemistry, were also prepared
by the PS-BEMP promoted cyclization of diacyl hydrazides and related compounds [66-
68]. In some cases, the use of microwave irradiation was extremely useful for facilitating
rapid access to the desired products [68].
A convenient
catch-cyclize-release
methodology based on the PS-BEMP mediated
sequestration was explored for the synthesis of 2-alkylthio-pyrimidinone derivatives
catch and release
catch
BEMP
+
H
1.
PS-BEMP
(1 equiv)
DMF, rt, 1 h, then 45 °C, 1 h
O
O
-
N
N
NH
2
+
R
2
S=C=N
R
1
R
2
N
H
R
1
N
H
2. wash resin
dioxane / H
2
O (1 : 1)
S
1. dioxane / H
2
O (1 : 1)
85 °C, 16 h
2. wash resin
MeCN (2 times)
cyclize
release
R
2
R
2
R
3
X (0.65 equiv)
-
N
N
R
3
S
R
1
R
1
HS
BEMP
+
H
MeCN, rt to 50 °C, 2 h
NN
NN
58-97% yield
Scheme 6.16
PS-BEMP mediated
catch-cyclize-release
preparation of 3-thio-1,2,4-triazole
derivatives