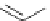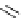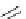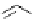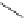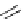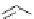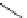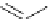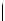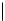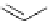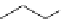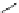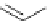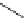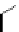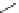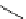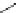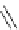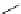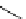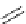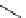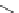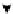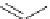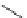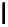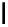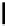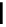Chemistry Reference
In-Depth Information
Ph
N
Ph
O
NNMe
2
Ph
NNMe
2
POPh
2
OEt
Me
N
Ph
Me
O
MeI in THF
Me
OHC
NO
2
NO
2
OEt
65%
P(NMe
2
)
3
84%
CHO
CH
3
CH
2
NO
2
in THF
NO
2
OH
O
H
Me
Me
+
NO
2
NO
2
O
2
N
O
2
N
70% (1.3 : 1)
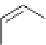
Scheme 6.4
C
C bond forming reactions using polymer-supported phosphorus ylide
This method is of great value in terms of its simplicity for purification as well as mild
reaction conditions and fast reactions. As a related example, a new type of polymer-
supported ylide base was found to be effective for the Henry reaction, the C-alkylation of
a
-amino acid derivatives, Wittig-olefination, and the N-alkylation of amide derivative
[32,33] (Scheme 6.4).
Fetterley et al. have been very active in determining the utility of strongly basic PAPT-
type reagents in organic synthesis, and have reported that
-aminonitrile compounds could
be efficiently synthesized by the Strecker condensation of aldehydes, amines, and
trimethylsilyl cyanide (TMSCN) in the presence of a nitrate salt of PS-PAPT as a
catalyst [34] (Scheme 6.5). In this work, the reusability of the catalyst was also established,
and the catalyst activity was ascribed to the participation of a nitrate ion of the catalyst acted
as a
a
The Michael addition reaction is one of the most important carbon-carbon bond forming
reactions in organic synthesis, and several examples using polymer-supported reagents
have been reported. For example, Bensa et al. found that the Michael addition reaction of
1,3-dicarbonyl compounds with activated olefins as a Michael acceptor could be efficiently
catalysed by PS-BEMP [35] (Scheme 6.6). The procedure does not require dry solvents or
an inert atmosphere, and filtration of the catalyst gives substantially pure products. It is also
proton shuttle.