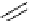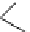Chemistry Reference
In-Depth Information
PS-TBD
(3 equiv)
ArOH +
ArOTf or ArONf
O
2
N
OX
MeCN, 80 °C
X = Tf or Nf (3 equiv)
Tf = SO
2
CF
3
; Nf = SO
2
C
4
F
9
Me
OX
XO
NHAc
t
Bu
OX
OX
OHC
OMe
COOMe
Me
X = Tf (65%)
X = Nf (81%)
X = Tf (quant)
X = Nf (98%)
X = Tf (95%)
X = Nf (96%)
X = Tf (89%)
X = Nf (83%)
Scheme 6.2
Triflation or nonaflation of phenols via sulfonate transfer reactions
Due to the importance of aryl triflates and related compounds in modern organic
chemistry, including metal catalysed cross-coupling reactions, an efficient procedure for
the triflation or nonaflation of phenols has been developed by the combination of PS-TBD
(3 equiv.) and a perfluoroalkanesulfonyl transfer reagent (3 equiv.) such as p-nitrophenyl
triflate or nonaflate [30] (Scheme 6.2). In this case, the free PS-TBD reagent could be
recovered by washing successively with diluted acid, base, water and organic solvents, and
almost comparable results were obtained with use of the recycled catalyst.
6.3 Alkylation Reactions
The solid base character of polymer-supported superbase reagents can be conveniently
applied to important carbon-carbon bond forming reactions. For example, PS-TBD has
been shown to be an efficient promoter to realize the addition reaction of nitroalkanes to
aldehydes and alicyclic ketones, that is, the Henry reaction [31] (Scheme 6.3).
R
1
R
1
OH
PS-TBD
(cat)
+
R
3
CH
2
NO
2
O
NO
2
R
2
R
2
R
3
R
3
= H or CH
3
OH
OH
Ph
OH
C
4
H
9
OH
NO
2
NO
2
NO
2
NO
2
H
H
Me
82% (0 °C, 1 h)
70% (0 °C, 5 h)
95% (0 °C, 5 min)
75% (0 °C, 1 h)
Scheme 6.3
Henry reactions of nitroalkanes with aldehydes or ketones