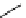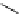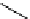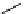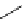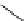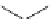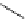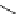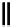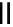Chemistry Reference
In-Depth Information
20 mol%
PS-PAPT
+
NO
3
-
CN
R
2
R
1
CHO
+
+
TMSCN
R
1
HN
R
3
NR
2
R
3
(1.2 equiv)
MeCN, rt
CN
CN
CN
Ph
H
H
Ph
N
O
99% (12 h)
99% (12 h)
99% (8 h)
CN
CN
Ph
Ph
H
Me
H
Cl
90% (25 h)
99% (15 h)
Scheme 6.5
Strecker-type condensation of aldehydes with amines and TMSCN
known that mesoporous silica materials or polymer-supported TBD could catalyse the
similar type of Michael addition reactions under remarkably mild conditions [36-38].
Surprisingly,
there have been only few synthetic studies on polymer-supported
superbase reagents. Recently, Wannaporn and Ishikawa prepared a new chiral
guanidine based polymer catalyst and applied it to the asymmetricMichael addition reaction
of iminoacetate with methyl vinyl ketone [39] (Scheme 6.7). Although the catalyst shows
only moderate levels of reactivity and enantioselectivity, the result demonstrates the
possibility of expanding an exciting field of asymmetric synthesis using polymer-supported
chiral superbase catalysts.
asymmetric
O
O
O
O
5 mol%
PS-BEMP
X
R
1
R
1
+
R
2
THF, rt
(1.1 equiv)
R
2
X
MeOOC
COOMe
O
O
O
O
O
O
CHO
COOMe
CN
COCH
3
COOMe
COCH
3
O
O
> 98% (8 h)
87% (24 h)
> 98% (23 h)
> 98% (4 h)
77% (72 h)
Scheme 6.6
Michael addition reactions of 1,3-dicarbonyl compounds