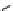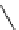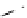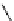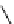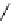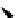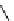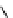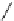Chemistry Reference
In-Depth Information
O
P
Me
Me
Me
N
Ar
Ar
P
Me
Me
N
rt
Me
N
N
2 Ar-CHO
+
+
benzene
O
N
N
Scheme 5.45
Diaryl epoxide formation from aryl aldehyde
5.4.2.6
Formation of Diaryl Epoxide
In contrast to its acyclic analogue P(NMe
2
)
3
, which in benzene at room temperature reacts
with two aryl aldehyde molecules bearing electron-withdrawing groups to give the
corresponding diaryl epoxides as an isomeric mixture (trans/cis ratio 72/28 - 51/49),
P(MeNCH
2
CH
2
)
3
N under the same reaction conditions is found to be a highly selective
reagent that provides epoxides with trans/cis ratios as high as 99/1. These reactions are
faster with the proazaphosphatrane because its phosphorus atom is apparently more
nucleophilic than that in P(NMe
2
)
3
[67] (Scheme 5.45).
5.4.2.7
-Hydroxynitrile Synthesis
The successful synthesis of
b
-hydroxynitriles was reported in good to excellent yields from
aldehydes and ketones in a simple reaction that is promoted by proazaphosphatrane bases.
The reaction occurs in the presence of magnesium salts, which activate the carbonyl group
and stabilize the enolate thus produced [68] (Scheme 5.46).
b
5.4.2.8
Stille Reaction
Proazaphosphatrane bases were used for palladium catalysed Stille reactions of aryl
chlorides. These bases efficiently catalyse the coupling of electronically diverse aryl
chlorides with an array of organotin reagents. The catalyst system based on benzyl (Bn)-
proazaphosphatrane is active for the synthesis of sterically hindered biaryls. The use of the
proazaphosphatrane allows room temperature coupling of aryl bromides and it also permits
aryl triflates and vinyl chlorides to participate in Stille coupling [69] (Scheme 5.47).
5.4.2.9
Palladium Catalysed Amination of Aryl Halides
The proazaphosphatrane bases serve as an effective ligand for the palladium catalysed
amination of a wide array of bromides and iodides. Other bicyclic or acyclic triaminopho-
sphines, even those of similar basicity and/or bulk, were inferior. The palladium catalysed
P
R
R
R
N
N
N
(10 mol%)
R
1
R
1
OH
O
+
CH
3
CN
CN
R
2
MgSO
4
R
2
Scheme 5.46
1,2-Addition of acetonitrile