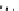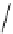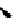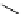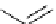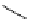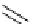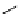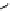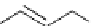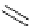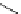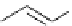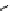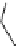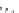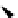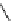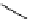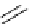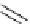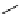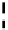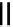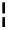Chemistry Reference
In-Depth Information
P
N
Ph
N
Ph
Ph
N
Cl
R'
R
+
R'
SnBu
3
R
Pd
2
(dba)
3,
CsF, dioxane
Scheme 5.47
Stille coupling using proazaphosphatrane ligand
amination reaction of aryl chlorides with amines also proceeded in the presence of the
i
Bu-
proazaphosphatrane to afford the corresponding arylamines in good to excellent yields.
Electron poor, electron neutral and electron rich aryl chlorides all participated with equal
ease [70] (Scheme 5.48).
5.4.2.10 Reduction with Silane
The CH
3
-proazaphosphatrane base can activate Si
H bonds in polymethylhydrosiloxane
(PMHS) to reduce carbonyl compounds. Aldehydes and ketones are reduced under mild
conditions by PMHS in the presence of the proazaphosphatrane, giving the corresponding
alcohols in high yield. A variety of aromatic aldehydes were smoothly reduced to the
corresponding alcohols in high yields with survival of the aromatic chloro, nitro, cyano and
methoxy substituents. Conjugated as well as isolated double bonds also remained intact
during regioselective reduction of the carbonyl groups [71] (Scheme 5.49).
i
Bu
i
Bu
P
i
Bu
N
N
R
2
N
X
N
R
2
R
3
+
HN
R
1
R
1
Pd(OAc)
2
R
3
NaO
t
Bu or LiN(TMS)
2
toluene
Scheme 5.48
Amination of aryl halides
P
Me
Me
Me
N
N
OH
N
O
Ph
Ph
Ph
Ph
PMHS, THF
Scheme 5.49
Reduction with silane