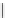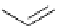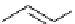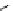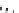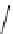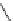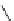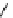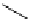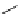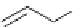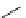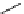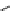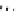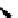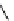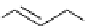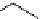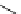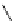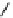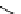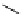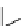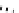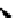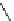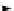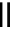Chemistry Reference
In-Depth Information
OR
4
O
R
4
OH
R
1
R
3
R
2
P
R
R
R
N
N
R
2
O
R
1
R
3
(cat)
N
R
4
R
5
O
2
N
O
R
4
R
1
R
3
R
2
NO
2
R
5
Scheme 5.42
1,4-Addition catalysed by proazaphosphatrane
catalysed direct
-arylation of nitriles with aryl bromides. Using the base, ethyl cyanoa-
cetate and primary as well as secondary nitriles are efficiently coupledwith awide variety of
aryl bromides possessing electron rich, electron poor, electron neutral and sterically
hindered groups [65] (Scheme 5.43).
a
5.4.2.5 Dimerization of Allylsulfone
In the presence of CH
3
-proazaphosphatrane catalyst, allyl phenyl sulfone readily dimerizes
to give the product shown, for which only incomplete and inconclusive data exist in the
literature. The dimer was shown from
1
H NMR spectral considerations to have the E
configuration [66] (Scheme 5.44).
i
Bu
i
Bu
P
i
Bu
N
N
R
1
R
2
N
Br
R
2
CN
R
1
R
1
+
CN
R
3
Pd(OAc)
2
LiN(TMS)
2
, toluene
Scheme 5.43
Arylation of substituted acetonitriles
P
Me
Me
Me
N
N
SO
2
Ph
N
(2.5mol%)
Me
SO
2
Ph
SO
2
Ph
MeCN, rt
Me
Scheme 5.44
Dimerization of allyl phenyl sulfone