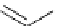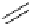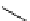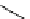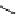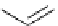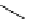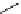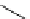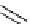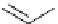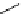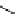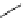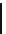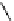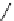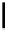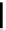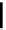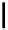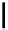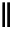Chemistry Reference
In-Depth Information
F
3
C
t
Bu-P4
R
2
CHO
SO
2
CH
2
R
1
R
1
CH=CHR
2
+
THF
F
3
C
Scheme 5.28
Julia-Kocienski olefination
R
2
CHO
R
2
t
Bu-P4
R
3
O
benzene (or pivalonitrile)
O
R
1
R
1
R
3
Scheme 5.29
Synthesis of benzofuran
5.2.3.5 Oxy-Cope Rearrangement
Compounds containing a 1,5-hexadien-3-ol system undergo anionic oxy-Cope rearrange-
ment when treatedwith the phosphazene superbase
t
Bu-P4. The sigmatropic rearrangement
occurs in hexane as well as in THF. The weaker phophazene base Et-P2 failed to induce
rearrangement. This is the first example of the use of a metal-free base to induce anionic
oxy-Cope rearrangement [49] (Scheme 5.30).
5.2.3.6 Ether Formation via S
N
Ar Reaction
In the presence of
t
Bu-P4 base and copper(I) bromide, aryl halides couple with phenols to
give biaryl ethers at about 100
C, while the use of DBU as a base for this coupling reaction
is not effective [50] (Scheme 5.31).
Ph
O
t
Bu-P4 (1.1 equiv)
OH
R
THF-hexane
Ph
R
R=H 44%
R=Ph 58%
Scheme 5.30
Anionic oxy-Cope rearrangement
R
R
I
HO
O
t
Bu-P4, CuBr (cat.)
R'
+
R'
toluene, reflux
Scheme 5.31
Copper catalysed coupling in the presence of P4