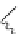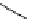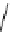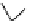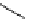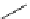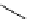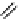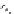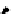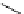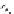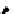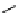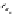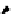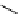Chemistry Reference
In-Depth Information
O
2
S
O
2
S
t
Bu-P4
Pr
Bn
Bn
BnBr
Pr
Pr
Pr
Pr
Pr
Scheme 5.26
Alkylation of episulfone in the presence of P4
Me
Bn
Me
H
Me
Me
O
Bn
O
H
N
N
t
Bu-P4
N
O
N
O
N
O
N
BnBr
O
Me
N
Me
N
O
Me
O
Me
Me
Bn
Me
Scheme 5.27
Benzylation of cyclic peptide
when
t
Bu-P4 was used as base. A further increase in the diastereoselectivity was observed
when the alcoholate was quenched with trimethylchlorosilane [46] (Table 5.1).
5.2.3.3 Julia-Kocienski Olefination
The reaction between a carbanion derived from alkyl 3,5-bis(trifluoromethyl)phenyl
sulfones and aldehydes affords, with good yields and stereoselectivities, the corresponding
1,2-disubstituted alkene through the Julia-Kocienski olefination reaction. This one-pot
protocol can be performed using the phosphazene base at
78
C and has been successfully
used in a high yielding and stereoselective synthesis of various stilbenes such as resveratrol
[47] (Scheme 5.28).
5.2.3.4 Benzofuran Cyclization
The hindered nonionic phosphazene base
t
Bu-P4 efficiently deprotonates o-arylmethoxy
benzaldehydes, leading to a direct synthesis of benzofuran derivatives. Strong ionic bases
such as LDA, LTMP and KH failed for this cyclization [48] (Scheme 5.29).
Table 5.1
Diastereoselective addition of sulfone to aldehyde
OH
OH
OO
OO
OO
Pr-CHO
S
Ph
S
S
+
Et
Et
Pr
Et
Pr
base
Ph
Ph
syn
anti
Base
anti
syn
Yield (%)
BuLi
59
41
100
t
Bu-P4
72
28
56
t
Bu-P4/TMSCl
82
18
72