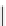Chemistry Reference
In-Depth Information
CH(OH)Ph
Br
Br
PhCHO
t
Bu-P4, ZnI
2
, THF
NC
NC
CH(OH)Ph
PhCHO
NN
NN
t
Bu-P4, ZnI
2
, THF
Scheme 5.32
Deprotonative functionalization of aromatic compounds
5.2.3.7 Reaction of Heteroaryl Carbanion
A novel type of deprotonative functionalization of aromatics was achieved with unique
regioselectivities and excellent chemoselectivity using
t
Bu-P4 base and zinc iodide (ZnI
2
)
in the presence of an electrophile [51] (Scheme 5.32).
5.2.4 Use of P5 Base
Pyrido[1,2-a]azepinone was deprotonated from the pyridine unit by lithium diisopropy-
lamide affording lithium salts, which were trapped by electrophiles like deuterium oxide
(D
2
O), benzoyl chloride and aldehyde. In the latter case, subsequent intramolecular attack
of the intermediate alkoxide to the lactam moiety leads to [7] (2,6)pyridinophanes. On
reaction with a lithium free phophazene base
t
Bu-P5 deprotonation takes place at the
a
-carbonyl position of the azepinone ring affording the enolate [52] (Scheme 5.33).
5.3 Transformation Using Phosphazene Catalyst
5.3.1 Addition of Nucleophiles to Alkyne
The addition of O- and N-nucleophiles to alkynes catalysed by a phosphazene base,
t
Bu-P4
base was investigated. Alkynes were easily transformed to enol ethers and enamines in
dimethyl sulfoxide (DMSO) by the addition of nucleophiles. When phenylacetylene was
Li
O
t
Bu-P5
O
LDA
O
N
N
N
Me
Me
Me
Scheme 5.33
Deprotonation using P5