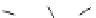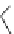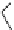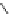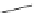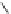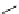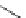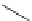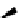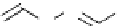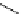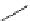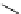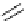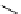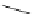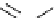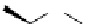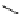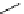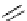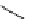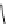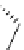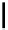Chemistry Reference
In-Depth Information
OTBDPS
OBn
BnO
OMe
BnO
TBDPSO
1. piperidine
O
O
BnO
BnO
TfO
BnO
+
S
O
O
BnO
BnO
O
2. BEMP
S
OBn
OBn
BnO
Me
OMe
OBn
Scheme 5.8
Synthesis of 1-thioglycoside
thio-dissacharides without substantial elimination product. The iminophosphorane bases
also proved to be useful in solid support-bound coupling of thioglycosides though with
lower efficiency [23] (Scheme 5.8).
5.2.1.4 Amide Formation
a
-Amino acids are soluble in acetonitrile when treated with phosphazene bases. As a result,
the protection/deprotection events that are usually used for peptide coupling reactions can
be minimized. This is illustrated in the synthesis of the important angiotensin-converting
enzyme (ACE) inhibitor enalapril [24] (Scheme 5.9).
5.2.1.5 Heterocycle Formation
3,6-Dihydro-2H-1,4-oxazin-2-ones act as reactive chiral cyclic alanine equivalents and can
be distereoselectively alkylated using the organic base BEMP when using unactivated alkyl
halides. Inmost cases, the diastereoselectivity is excellent although the reactions are always
carried out at room temperature. Hydrolysis of the alkylated oxazinones obtained allows the
preparation of enantiomerically enriched (S)-
a
-methyl
a
-amino acids. The organic base
methodology has also been applied to the synthesis of (R)-
-amino acids starting
from (R)-alanine. When dihalides are used as electrophiles in the presence of BEMP, a
spontaneous N-alkylation also takes place giving bicyclic oxazinones, which can be
hydrolyzed to an enantiomerically pure heterocyclic compound [25] (Scheme 5.10).
a
-methyl
a
Ph
COOEt
Ph
COOEt
COOH
H
HN
Me
HN
Me
t
Bu-P1
MeCN
ON
rt
OO
N
e
HOOC
Me
Scheme 5.9
Peptide coupling reaction
Me
Me
I
I
O
O
O
O
Me
Me
Me
BEMP
NMP
Ph
N
Ph
N
Me
Scheme 5.10
Alkylation of chiral alanine equivalent