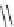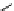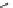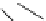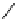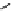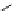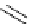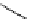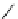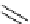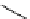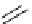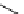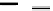Chemistry Reference
In-Depth Information
O
O
O
O
COOEt
COOEt
Ph
N
P
NMe
2
3
(10 mol%)
>99%
H
2
O, rt
Scheme 5.11
1,4-Addition reaction to enone
5.2.1.6 Michael Addition
Michael addition reaction of various
-ketoesters with several Michael acceptors in water
containing 10mol% of N-phenyl-tris(dimethylamino)iminophosphorane results in high
yield conversion to the corresponding adducts [26] (Scheme 5.11).
b
5.2.1.7
Polymer-Supported Reaction
The polymer bound indolecarboxylate was N-alkylated with 3-cyanobenzyl bromide in the
presence of the strong base BEMP (Scheme 5.12). The replacement of the base by the
weaker base DIPEA did not lead to any alkylation product [27].
Polymer bound acrylic ester is reacted in a Baylis-Hillman reaction with aldehydes to
form 3-hydroxy-2-methylidenepropionic acids or with aldehydes and sulfonamides in a
three-component reaction to form 2-methylidene-3-[(arylsulfonyl)amino]propionic acids.
In order to show the possibility of Michael additions, the synthesis of pyrazolones was
chosen. The Michael addition was carried out with ethyl acetoacetate and BEMP as base to
form the resin bound
b
-keto ester. This was then transformed into the hydrazone with
phenylhydrazine hydrochloride in the presence of TMOF and DIPEA [28]. The polymer
bound phenol was readily coupled to a variety of allyl halides by using the P1-
t
Bu to
generate a reactive phenoxide [29].
Polymer-supported reagents have been applied to the synthesis of the natural product
carpanone, resulting in a clean and efficient synthesis without the need for a conventional
Br
X
COOEt
CN
X
N
COOEt
BEMP
DMF
H
CN
Scheme 5.12
Alkylation of immobilized indole