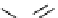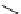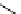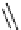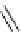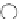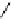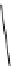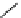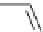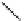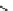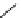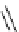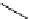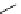Chemistry Reference
In-Depth Information
N
t
Bu
N
P
N
O
2
N
3
CN
CH
2
COOEt
N
COOEt
H
H
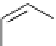
Scheme 5.5
Synthesis of pyrrole derivative from nitroarene
NMe
2
Me
Me
N
OAc
BEMP
OMe
OMe
AcO
N
Ph
O
OCONHPh
O
Scheme 5.6
Intramolecular alkylation for cyclization
5.2.1.2 Alkylation and Arylation of N-Nucleophile
The cyclization associated with the quasi-axial orientation of the carbamate residue
involves complications, not unexpected because of the steric/stereoelectronic conditions.
After a series of inadequately selective reactions with a variety of conventional bases (NaH,
KH, LDA,
t
BuOK, etc.) the Schwesinger iminophosphorane bases eventually enabled a
breakthrough and gave the desired product in high yield [21] (Scheme 5.6).
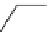
The amination of 3-bromoisoxazoles by a nucleophilic aromatic substitution reaction
was facilitated by the use of phosphazene bases. 3-Bromoisoxazoles were found to be inert
to substitution under thermal conditions. However, the use of phosphazene bases under
microwave irradiation facilitates the amination process and allows the corresponding
3-aminoisoxazoles to be isolated in moderate yield [22] (Scheme 5.7).
5.2.1.3 Alkylation of S-Nucleophile
Disaccharides of 1-thioglycosides, an important class of glycomimics, can be synthesized
by S-alkylation in exceptionally high yields when iminophosphorane bases are employed.
The reaction conditions employed appear to be general and stereospecific. Axial and
equatorial 4-triflates and primary tosylates of alkyl pyranosides provided excellent yields of
Br
BEMP
N
+
N
MW
MeCN
n
Bu
O
H
N
n
Bu
O
Scheme 5.7
Amination of 3-bromoisoxazole