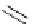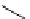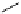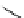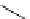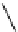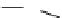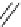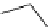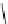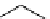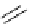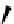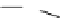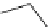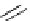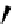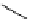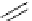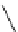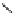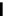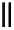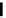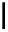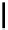Chemistry Reference
In-Depth Information
CO
2
H
HO
Me
H
H
O
Me
OP(O)(OPh)
2
N
+
HS
N
O
N
+
H
H
Cl
-
CO
2
R
H
R = CH
2
Ph(NO
2
)-4
N
-ethylpyrrolidine
1 h, -60 ˚C
TMG (
1
)
CO
2
-
HO
Me
O
H
H
Me
N
S
N
N
H
TMGH
+
O
H
CO
2
R
Scheme 4.27
TMG (1) assisted substitution reaction for the practical preparation of ertapenem
sodium
4.3.3 Others
4.3.3.1 Construction of Heterocycles
Benzimidazole
Copper and palladium catalysed intramolecular C-N bond formation between an aryl
halide and a guanidine moiety affords 2-aminobenzimidazoles. Inexpensive copper salts
such as copper iodide (CuI) are generally superior to the use of palladium catalysts [76]
(Table 4.12).
Furan
Effective cyclization of 2-trimethylsilylethynylphenol to a 2-trimethylsilylbenzfuran was
carried out by refluxing in toluene in the presence of TMG (1)(
90%) (Scheme 4.28). The
co-presence of silicon dioxide (SiO
2
) led to a desilylated benzofuran in one pot [77].
>
Oxazolidinone and Oxazole
N-Alkylprop-2-ynylamines readily react with carbon dioxide (CO
2
) in the presence of
catalytic strong bases and undergo intramolecular cyclization to 5-methylene-1,3-oxazo-
lidin-2-ones in good yields [78]. The type and strength of the base is of paramount
importance to the success of the reaction. DBU, TBD, tetra-alkyl- and penta-alkylguani-
dines and phosphazene bases are effective, whereas 1.8-bis(dimethylamine)naphthalene
(proton sponge), carbodiimide and pyridine did not work. The presence of a triple bond and
an amino group, which can react intramolecularly, allow the catalytic incorporation of the
intermediate carbamate into an oxazolidine ring by reaction with the triple bond even
without any metals (Scheme 4.29a).