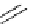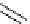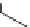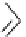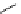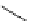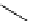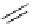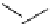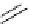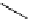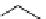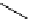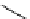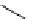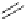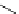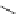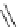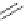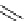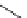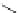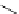Chemistry Reference
In-Depth Information
Table 4.12
Intramolecular aryl guanidinylation of aryl bromides with Pd(PPh
3
)
4
or CuI
R
1
R
1
Br
cat. A or cat. B
a
R
2
N
N
R
4
R
4
N
R
2
N
R
3
N
H
N
1,10-phenanthroline (10 mol%)
R
3
Cs
2
CO
3
(2 equiv)
Yield (%) [conversion (%)]
R
1
NR
2
R
3
R
4
Run
cat. A
cat. B
N
1
Bn
H
88 (
98)
83 (
98)
>
>
N
2
Ph
H
84 (
95)
58 (
95)
>
>
N
3
Bn
H
93 (
98)
96 (
98)
>
>
NBoc
Me
4
Bn
H
(85)
97 (
98)
>
N
H
N
5
Bn
4-Me
66 (70)
90 (95)
N
6
Bn
6-Br 4-Me
76 (76)
98 (
98)
>
a
cat. A: Pd(PPh
3
)
4
(10mol%); cat. B: CuI (5mol%).
PS-p-toluenesulfonylmethyl isocyanide (TosMIC) reagent, developed by Barrett et al.
was found to be effective for the conversion of a range of aryl aldehydes into highly pure
4-aryl oxazoles in the presence of BTMG (2) [79] (Scheme 4.29b). A typical procedure
involved the reaction of aldehyde with the gel (4 equiv.) in acetonitrile (0.2M sol) and
BTMG (2) (4 equiv.) for 12 h at 65
C.
SiMe
3
SiMe
3
OH
O
O
MeO
2
C
TMG (
1
)
MeO
2
C
MeO
2
C
SiO
2
, toluene
NHAc
NHAc
NHAc
Cl
Cl
Cl
Scheme 4.28
TMG (1) mediated intramolecular cyclization to furan ring system