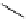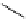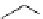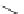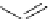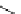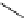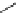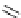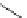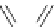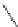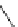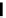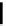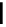Chemistry Reference
In-Depth Information
R
1
R
2
O
O
R
2
(EtO)
2
P
R
1
O
(ex) R
1
= Me, R
2
= OMe (51%)
R
1
= H, R
2
= Me (54%)
O
TMG (
1
)
(EtO)
2
P
H
O
(EtO)
2
P
CN
CN
47%
Scheme 4.19
TMG (1) catalysed Michael addition with phosphites
Thio-Michael Reaction
g
-nitroalcoholsweresmoothlypreparedbyTMG(1) catalysed one-pot
reaction of nitroolefines, thiophenol and aldehydes [60]. During the course of the
synthesis of ecteinascidins [61] (Scheme 7.20), the ten-membered lactone bridge
through the sulfide bond formation, based on Corey
-Phenylthio-
b
s original method, was achieved
by BTMG (2) promoted intramolecular Michael type addition of thiolate ion to quinone
methides, which were produced by treatment with Tf
2
OinDMSOfollowedbyH
unig
€
base (Scheme 4.20).
4.3.1.9 Nucleophilic Epoxidation
Novel guanidine bases supported on silicas and micelle-templated silicas have been
prepared and investigated in the base-catalysed epoxidation of election-deficient alkenes.
OMe
MeO
Me
OMe
allyl-O-COHN
1. Tf
2
O
2.
I
Pr
2
NEt
3.
t
BuOH
MeO
Me
O
O
H
OH
Me
HO
Me
S
H
N
O
Me
Me
N
N
4. BTMG (
2
)
5.
Ac
2
O
O
N
O
CN
O
O
O
CN
O
S
79%
H
allyl-O-COHN
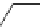
Scheme 4.20 The use of the BTMG (2) catalysed intramolecular Michael addition as a key step
for ecteinascidin synthesis