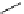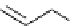Chemistry Reference
In-Depth Information
reaction of 1,3-dicarbonyl compounds with a broad range of conjugated nitroalkanes and
shows extremely high catalytic activity [54]. The catalyst showed high catalytic efficiency
in evaluation by a gram-scaled experiment with low catalyst loading and was recovered in a
nearly quantitative yield as an hydrochloride salt by acidic work up following column
purification.
A chiral bicyclic guanidine, which corresponded to the tert-butyl analogue of guanidine
12 used in Scheme 4.12, effectively catalysed Michael reactions of dithiomalonates and
b
-keto thioesters using a range a of acceptors including maleimides, cyclic enones,
furanone and acyclic 1,4-dicarbonyl butenes [55]. TMG (1) immobilized silica gel was
used as a basic agent for the Michael addition of cyclopentenone and nitromethane.
Addition product was obtained in good yield under mild conditions and the catalysis
activity was maintained after fifteen cycles (98% conversion) [56].
Oxa-Michael Reaction
A 2,2-disubstituted chromane system was asymmetrically constructed by application of
intramolecular oxa-Michael addition reaction through 6-exo-trig mode cyclization [57].
Good asymmetric induction at the quaternary carbon was observed when Z-alkene was
treated with the same guanidine 17 used in asymmetric carba-Michael reaction in Table 4.5
(Scheme 4.18).
Phospha-Michael Reaction
Similar to aldol-type reaction, dialkyl phosphites [28] can also serve as good nucleophiles in
Michael reactions in the presence of TMG (1) (Scheme 4.19). The reaction proceeds
smoothly under mild conditions and shows tolerance to variety of functional groups.
A highly enantioselective 1,4-addition reaction of nitroalkene with diphenyl phos-
phite was successfully accomplished using an alternative axial guanidine catalyst 18b
with an internal guanidine unit [58] (Figure 4.6). A broad range of nitroalkenes, bearing
not only aromatic but also aliphatic substituents, is applicable to the present enantio-
selective reaction. A chiral bicyclic guanidine, which corresponds to the tert-butyl
analogue shown in the reaction (Scheme 4.12), has been used to catalyse the phospha-
Michael reaction of diarylphosphine oxide to nitroalkenes with high enantioselectiv-
ities, offering a direct methodology to prepare chiral
b
-aminophosphine oxides and
b
-aminophosphines [59].
ent
-17
(0.2 equiv)
CO
2
Me
CO
2
Me
CHCl
3
OH
O
Me
Me
rt, 2 d
ent
-17a
: (
S
)-product in 58% (32% ee) from
E
-deriv.
ent
-17a
: (
R
)-product in 75% (71% ee) from
Z
-deriv.
ent-17b
: (
R
)-product in 83% (70% ee) from
Z
-deriv.
Scheme 4.18
Guaniodine catalysed intramolecular oxa-Michael addition