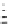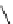Chemistry Reference
In-Depth Information
the Murphy's PTC
(0.05 equiv)
99% (93% ee)
Me
NaOCl, PhMe
O
N
Ph
O
Ph
Ph
H
NN
H
Ph
Ph
O
19
(0.2 equiv)
(1 equiv)
Ph
Ph
90% (70% ee)
19
70% cumene hydroperoxide
in toluene (10 equiv)
Scheme 4.21
Examples of nucleophilic epoxidation of chalcone
Excellent conversions and selectivities were observed both with respect to the alkene and
the primary oxidant [62]. In nitro-Michael reaction it was noted that theMurphy
s PTC does
not work as a good chiral catalyst for the Michael reaction of chalcone, but the same PTC
effectively catalyses the epoxidation of chalcones with sodium hypochlorite (NaOCl)
[24c]. Trials for the epoxidation of chalcone in the combination of hydroperoxides and
modified guanidines 19 [27b] resulted in less effective asymmetric induction compared to
the Murphy
s PTC [53] (Scheme 4.21).
4.3.1.10
Strecker Reaction
A diketopiperazine 8 with an external guanidine function is proven to be an effective
catalyst for the Strecker reaction of benzhydrylimine [14]. Corey
s bicyclic guandine 20
[12b], which is the original of 12 (Scheme 4.12), also works well in the same reaction and
the mode of action has been elucidated by density functional theory [63] (Scheme 4.22).
A review elsewhere discusses catalytic Strecker
reactions including guanidine
catalysts [64].
8
(0.02 equiv)
R = H, Ph; Ar = aryl
80-98% (64-99% ee)
MeOH, -25 ºC, 20 h
Ph
R
N
CN
R
H
+
Ar
1
Ar
2
HCN
(2 equiv)
N
N
N
Ar
1
Ar
2
N
H
(1 equiv)
Ph
20
20
(0.1 equiv)
Ar
1
= R = Ph; Ar
2
= aryl
80-99% (50-88% ee)
toluene, -40 ºC, 20 h
Scheme 4.22
Guanidine catalysed Strecker reaction