Environmental Engineering Reference
In-Depth Information
At the equilibrium potential, some steps are uphill in free energy, meaning that the
reaction on the surface is slow. A perfect catalyst in this analysis would be characterized
by a flat potential energy landscape at the equilibrium potential, i.e., by all steps having
the same height at zero potential. Whereas no such catalyst has yet been found, we can
define the highest potential at which all steps are just downhill in free energy, U
ORR
Max
.
Max
, we would say that the reaction starts to be transport-limited. At potentials
above U
ORR
Below U
ORR
Max
, the catalytic reactions on the surface are limiting. The higher U
ORR
Max
is the
Max
better the catalyst, and, as mentioned above, for the perfect material, U
ORR
corresponds
exactly to the equilibrium potential.
This analysis only deals with the change in free energy for each reaction step. To do
better, we would have to make detailed calculations of the barrier for proton and
electron transfer between the surface and the electrolyte. At present, this is too com-
putationally demanding. Another challenge associated with that approach is how to
connect the output of such calculations with the standard hydrogen electrode in a
rigorous manner. The reason why the simple no-barrier analysis is still valid is
linked to the fact that the transition state often scales with the change in free energy
of the reaction; this is the so-called Brønsted - Evans - Polanyi relation found through-
out surface catalysis [Nørskov et al., 2002].
In Fig. 3.5, we consider the reaction free energy diagram for Pt(111) at different
potentials. As can be seen, some steps have larger free energy changes than others,
meaning that Pt is not a perfect catalyst for the ORR. Atomic oxygen, O
, and
hydroxyl, OH
, seem to be too strongly bound to the Pt surface, whereas peroxide,
OOH
, is too weakly bound. This can also be seen looking at the free energy surface
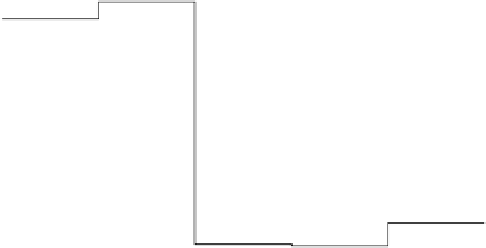
at a realistic potential of 0.9 V (Fig. 3.6). In this graph, two reactions are uphill: the
formation of OOH
and the reaction of OH
.
It is important to realize that the binding energies of O
,OH
, and OOH
most likely
cannot be varied independently by changing the catalyst. When looking deeper into the
Figure 3.6 The free energy surface at U ¼ 0.9 V; the two uphill reactions, O
2
(g)
!
OOH
and OH
!
H
2
O(l ), are seen directly.



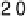
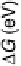

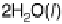


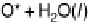

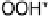
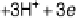
















Search WWH ::

Custom Search