Environmental Engineering Reference
In-Depth Information
3.4 CATALYTIC ACTIVITY: THE OXYGEN REDUCTION REACTION
3.4.1 Reaction Mechanism
As with the phase diagrams and Pourbaix diagrams, the theoretical standard hydrogen
electrode also allows us to calculate the relative energies of intermediates in electro-
chemical reactions. As an example, we investigate the oxygen reduction reaction
(ORR). We look at the four proton and electron transfer elementary steps:
O
2
þ
þ
(H
þ
þ
e
)
!
HO
2
(3
:
14)
HO
2
þ
(H
þ
þ
e
)
!
H
2
O
þ
O
(3
:
15)
O
þ
(H
þ
þ
e
)
!
HO
(3
:
16)
HO
þ
(H
þ
þ
e
)
!
H
2
O
þ
(3
:
17)
This analysis cannot tell whether or not atomic oxygen is formed on the surface during
the ORR. One could imagine alternative reaction pathways through H
2
O
2
, but O
is a
more stable intermediate, and since our analysis will show that either the formation of
HO
2
or the formation of water is potential-determining, the conclusions will not
change if the reaction goes through a hydrogen peroxide intermediate.
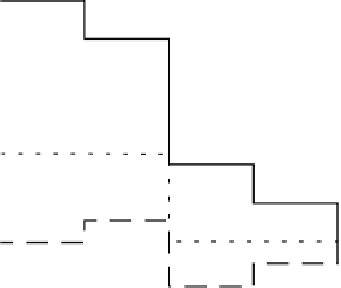
3.4.2 Potential Energy Surface
Based on the theoretical electrochemistry method outlined above in combination with
DFT calculations, the potential energy of the intermediates can be obtained at a given
potential, (Fig. 3.5). Since all steps involve exactly one proton and electron transfer,
the height of the different steps scales directly with the potential. To calculate the
potential energy landscape at the equilibrium potential, the levels are moved down
by n
1.23 eV, where n is the number of the electrons at the given state (the horizontal
axis in Fig. 3.5).
Figure 3.5 The free energy of the intermediates along ORR on Pt(111) at three potentials:
U ¼ 0 V with respect to a standard hydrogen electrode, the equilibrium potential U ¼ 1.23 V,
and the highest potential where all steps are downhill in free energy, U ¼ U
ORR
Max
¼ 0.78 V.








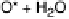

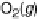
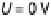
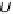
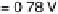














Search WWH ::

Custom Search