Environmental Engineering Reference
In-Depth Information
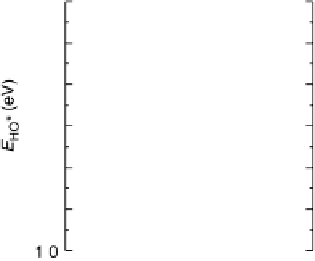
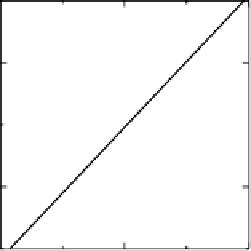
Figure 3.7 (a) Adsorption energy of HO
as function of the adsorption energy of O
, both on
the terrace. The best linear fit is E
HO
¼ 0 .50E
O
þ
0.05 eV. (b) Adsorption energy of HOO
as
function of the adsorption energy of O
, both on the terrace. The best linear fit is E
HOO
¼
0.53E
O
þ
3.18 eV.
correlations between binding energies on transition metal surfaces, it turns out that the
binding energies are, in fact, linearly dependent [Abild-Pedersen et al., 2007]. A
material with a strong binding of OH
can be expected to have a strong binding of
O
and OOH
as well. In Fig. 3.7, the binding of OH
is shown as function of the bind-
ing of O
, and a similar relationship is shown for OOH. Since what we need is a catalyst
where all steps have equal free energy barriers, when looking at transition metal
electrodes, we can already at this stage tell that there will be intrinsic limitations.
When put into an appropriate model [Nørskov et al., 2004], the binding energy cor-
relations directly define a limit to U
ORR
Max
on the metals obeying the linear relations
shown in Fig. 3.7. Since all intermediates are dependent on E
O
, it is possible to plot
the heights of all the steps DG
1-4
as functions of E
O
at zero potential. The step
with the smallest free energy change will define U
ORR
Max
(Fig. 3.8):
U
Max
ORR
¼
Max[
DG
1
(DE
O
)
=
e,
DG
2
(DE
O
)
=
e,
DG
3
(DE
O
)
=
e,
DG
4
(DE
O
)
=
e]
(3
:
18)
The first proton transfer, DG
1
, to oxygen forming OOH
defines the limit to U
ORR
Max
for
weakly binding metals such as Au, whereas DG
4
, the proton transfer to OH forming
water, defines the limit to U
URR
Max
for the strongly binding metals. Notice that Pt is found
close to the top of the “volcano,” meaning that, among the pure metal (111) surfaces,
Pt is the best catalyst for the ORR.
This illustrates the Sabatier principle: a good catalyst is a material with an optimal
trade-off between being reactive (strong binding of intermediates) and not being
poisoned by reaction products (weak binding). Obviously, this principle also
holds for electrocatalysts, and, using the linear relations between the binding of

















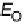
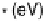
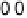
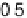
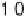
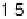








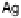
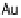





















Search WWH ::

Custom Search