Environmental Engineering Reference
In-Depth Information
electrolyte solution at an RDE cell open to the atmosphere at elevated temperatures. In
this section, we report the kinetically controlled current density j
k
for the HOR at Pt,
Pt-Co, and Pt-Ru planar electrodes with and without CO poisoning as a function of
temperature from 30 to 90 8C [Uchida et al., 2006]. j
k
is considered as a clear target
that one can achieve in the ideal case.
We employed a channel flow cell, which can be operated under a closed system
with controlled [H
2
] and can provide precise j
k
from hydrodynamic voltammograms.
The working electrode of Pt, Pt-Co, or Pt-Ru was prepared on a mirror-finished Au
substrate (1 mm
4 mm) by DC sputtering. The active area of the working electrode
S
Ft
was determined from the DQ
H
in the CV in N
2
-purged 0.1 M HClO
4
solution,
where the superscript “o” denotes the CO-free surface. The CO coverage u
CO
was
calculated from Equation (10.2) after CO was adsorbed at 0.05 V by supplying a sol-
ution saturated with 0.1% CO (H
2
balance) for various time intervals. Hydrodynamic
voltammograms for the HOR at various working electrodes were recorded under a
flow of H
2
-saturated (CO-free) solution (mean flow rate U
m
¼ 10 - 50 cm s
21
)from
0 to 0.10 V at the sweep rate of 0.5 mV s
21
.
The kinetically controlled current I
k
at 0.02 V was determined from a well-defined
equation [Levich, 1962; Gerischer et al., 1965], i.e., plotting the inverse of the current
I
21
against U
1
=
m
and extrapolating U
1
=
m
to zero. j
k
was obtained as I
k
=
S
Pt
. The
value of the apparent rate constant k
app
for the HOR was calculated from the equation
j
k
=
2F
¼
k
app
[H
2
]
(10
:
4)
where [H
2
] was determined from the limiting current and the diffusion constant.
Figure 10.8 shows Arrhenius plots of k
app
at 0.02 V for CO-free electrodes. The
Figure 10.8 Arrhenius plots for the apparent rate constant k
app
for the HOR (CO-free) at Pt
(W), Pt
51
Co
49
(D), and Pt
54
Ru
46
(A) working electrodes at 0.020 V vs. RHE(t). (From
Uchida et al. [2006], reproduced by permission of the American Chemical Society.)

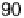




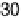












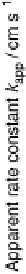



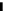
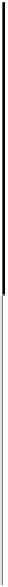







































Search WWH ::

Custom Search