Environmental Engineering Reference
In-Depth Information
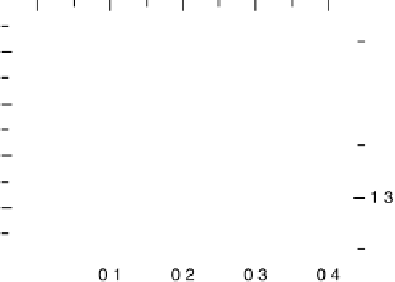
Figure 10.7
Linear relation between CL shifts (DCL) and DSCLS or CO adsorption energy
E
ads
(CO).
(From
Wakisaka
et
al.
[2006],
reproduced
by
permission
of
the
American
Chemical Society.)
Shek et al., 1982; Bj ¨ rneholm et al., 1994; Cabeza et al., 1999]:
E
ads
(CO/Pt)
¼
DSCLS
þ
E
ads
(CO/Au)
(10
:
3)
Figure 10.7 shows a plot of DSCLS, as a measure of E
ads
(CO), as a function of DCL.
E
ads
(CO) is found to decrease linearly with increasing DCL. The Pt - CO bond strength
is evidently weaker on the alloy electrodes than on pure Pt. Thus, the HOR cannot be
hindered by such a weakly bonded CO at the alloys. It should be noted that Ru is
a more effective element in weakening the bond strength than Co. Because Pt-Ru
exhibited a larger DCL than as-prepared Pt-Co (with Co remaining near the Pt site),
as evidenced in Fig. 10.4a, the exact location of such second metals is not essential
for the modification, as long as the skin layer is thin enough.
This is the first experimental demonstration of changes in the strength of CO
adsorption at Pt-based alloy electrodes. Nørskov and co-workers theoretically
predicted a similar linear relation between changes in E
ads
(CO) and shifts in the
d-band center [Hammer et al., 1996; Hammer and Nørskov, 2000; Ruban et al.,
1997]. Because the Pt4f
7/2
CL shift due to alloying can be more easily measured
by XPS than the d-band center can, this should be one of the most important
parameters to aid in discovering CO-tolerant anode catalysts among Pt-based alloys
or composites.
10.2.4 Temperature Dependence of CO-Tolerant HOR
Activity at Pt, Pt-Co, and Pt-Ru
Besides using Pt-Ru or Pt-Co alloy anodes, CO poisoning can be mitigated by elevat-
ing the operating temperature. However, temperature dependencies of the HOR rates
in the presence of CO with relevance to PEFC operation have been scarcely reported.
One of the difficulties is correction of the change in H
2
concentration [H
2
]inthe













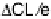


























Search WWH ::

Custom Search