Environmental Engineering Reference
In-Depth Information
value of k
app
at a pure Pt electrode is the highest in the whole temperature range. Pt-Co
and Pt-Ru exhibited almost comparable k
app
at 30 - 50 8C. The apparent activation
energies at Pt, Pt-Co, and Pt-Ru at 30 - 70 8C were as low as 3.0, 4.2, and 5.3 kJ
mol
21
, respectively. Hence, elevating the operation temperature does not lead to sig-
nificant enhancement in HOR kinetics. For example, k
app
for the HOR on Pt at 90 8C
was two times higher than that at 30 8C, whereas k
app
for the ORR on Pt increased by
about one order of magnitude from 30 to 90 8C [Wakabayashi et al., 2005a], as
described in the next section.
Next, the j
k
values in the presence of CO
ad
at these electrodes are compared.
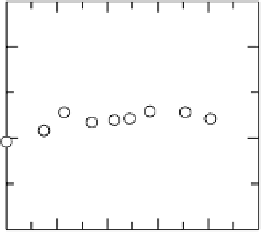
Figure 10.9 shows the current per remaining hydrogen adsorption site free from
CO
ad
, j
H
k
¼
j
k
=
(1
u
CO
), as a function of u
CO
. If the HOR active sites are tightly
blocked by the immobile CO
ad
, the value of j
H
k
should be a constant independent
of u
CO
. j
H
k
at pure Pt increases slightly up to u
CO
¼ 0.6, but it decreases steeply at
high u
CO
. Such an increase in j
H
k
at low u
CO
suggests that Pt sites were blocked not
rigidly by CO
ad
at least for u
CO
, 0.6, resulting in apparent active sites more than
that expected from u
CO
. The steep decrease in j
k
or j
H
k
at high u
CO
.
3
is certainly
due to a decrease in nearest neighbor CO-free pair sites required for H
2
dissociative
adsorption in the rate-determining Tafel step [Conway and Tilak, 2002; Uchida
et al., 2006].
Figure 10.9 Dependence of j
H
k
¼ j
k
/(1 2 u
CO
) at 0.020 V on u
CO
for Pt, Pt
51
Co
49
, and
Pt
54
Ru
46
electrodes in H
2
-saturated 0.1 M HClO
4
solution. (From Uchida et al. [2006], repro-
duced by permission of the American Chemical Society.)


















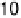
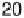


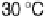
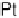


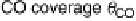








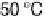

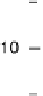





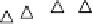





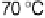
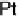

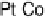

























Search WWH ::

Custom Search