Chemistry Reference
In-Depth Information
7.3.1.4 evaluating specificity during sIm development
Another key parameter to evaluate during SIM development is specificity. The USP
and various ICH guidelines define specificity as the ability of a method to unequivo-
cally assess the analyte of interest in the presence of potential interferences (1, 2). In the
past, it was acceptable to evaluate resolution, peak shape, and tailing factors to measure
and document specificity. However, starting with USP 25, and as a direct result of the
ICH process, it was recommended that a peak purity test based on photodiode array
(PDA) detection or mass spectrometry (MS) be used to demonstrate that a given peak
was pure—that nothing co-elutes.
Modern PDA technology is a powerful tool for evaluating specificity. PDA detec-
tors can collect spectra across a range of wavelengths at each data point collected
across a peak, and through software manipulations involving multidimensional vec-
tor algebra, compare each of the spectra to determine peak purity. In this manner,
PDA detectors today can distinguish minute spectral and chromatographic differ-
ences not readily observed by simple overlay comparisons [18-20]. To be successful,
three components are required:
1. A UV chromophore, or some absorbance in the wavelength range selected
2. Some degree of chromatographic resolution
3. Some degree of spectral difference
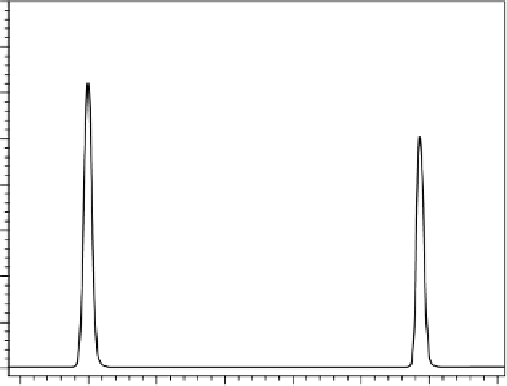
Figure 7.4 shows an example of a partial reversed-phase LC separation, where, by all
appearances, the peaks are certainly well resolved, sharp, and symmetrical.
An examination of peak two indicated the peak was pure. However, a close exam-
ination of the spectral information related to peak one reveals a different situation.
0.80
0.70
Peak6 - 16.986
0.60
Peak7 - 21.862
0.50
0.40
0.30
0.20
0.10
0.00
16.00
17.00
18.00
19.00
20.00
21.00
22.00
23.00
Minutes
FIgure 7.4
Example PDA chromatogram used to evaluate specificity/peak purity.