Chemistry Reference
In-Depth Information
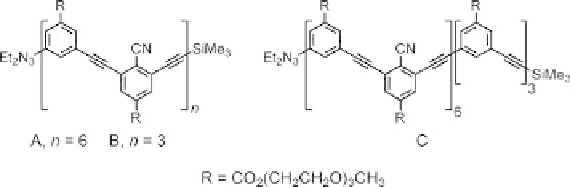
Figure 11.2 Abiotic oligomers for the examination of solvophobic and coordination interac-
tion [11].
binding. This oligomer contains six cyano groups located on alternating aromatic rings
that are available for metal coordination (Figure 11.2, oligomer A).
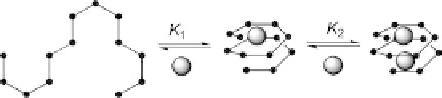
In the helical conformation, this sequence places the six cyano groups into the interior
of the tubular cavity, creating two trigonal planar coordination sites (Figure 11.3). The
solvent of choice for metal-binding experiments was tetrahydrofuran, which does not
cause a solvophobically driven helical structure in this system [11]. The metal selected
was silver triflate (AgO
3
SCF
3
) because it can adopt a trigonal planar coordination geome-
try [12]. Changes in UV-vis spectra upon metal binding were indicative of a cisoid confor-
mation of the diphenylacetylene units, consistent with a helical structure [11], which was
further confirmed by
1
H-NMR spectroscopy.
In addition, the UV titration spectra did not change after two equivalents of AgO
3
SCF
3
were added, indicating that two Ag
þ
ions were bound to each oligomer. The association
constant of the overall reaction (
K
1
K
2
) was estimated to be greater than 10
12
M
2
. In order
to further investigate the binding reaction, oligomers B and C (Figure 11.2), anticipated to
bind one equivalent of AgO
3
SCF
3
, were synthesized and tested for metal coordination.
UV-vis,
1
H-NMR and ESI-MS spectra confirmed that only oligomer C binds to silver
triflate, with an association constant of
K
1
¼
10
4
M
1
. These results suggest that the
binding of two equivalents of AgO
3
SCF
3
in oligomer A is a cooperative process with
K
2
2
K
1
(Figure 11.3). Overall, this work demonstrates that folding is driven by a combi-
nation of solvophobic interactions that favor the helical structure and metal-ligand inter-
actions. Hence, the oligomer can be modified to selectively bind metal ions as it templates
the exterior turns of the helical structure, and consequently non-covalent interactions
nucleate the formation of a central turn in the structure leading to a non-biological single-
stranded helix.
While the above example involves linear oligomers, large macrocycles also can
form helical complexes upon metal binding. Coordination to a metal ion forces the
Figure 11.3 Representation of the metal-induced formation of helical structures as reported
by the group of Moore. The metal ions (Ag
þ
) are shown as spheres [11].
Search WWH ::

Custom Search