Chemistry Reference
In-Depth Information
macrocycle to adopt a twisted conformation [13], in which its two halves create a
double helical system. In rare cases, two diastereomeric structures of opposite helic-
ity can be obtained for one compound by a thermodynamic inversion process. While
helix inversion [14,15] between well defined and well characterized diastereomers is
a biological phenomenon [16] found in natural systems, a similar process is not
common in artificial systems. Following the idea that enantiomerically pure ligands
will lead to metallofoldamers with a single-handed helical structure, Muller and
Lisowski reported a chiral nonaazamacrocycle amine, which coordinates Ln
3þ
ions
to form enantiopure helical complexes (Figure 11.4) [17]. Moreover, helix inversion
between the kinetic and thermodynamic binding products in the Yb
3þ
complexes
was also demonstrated. The nonaaza macrocycle
L
was prepared by the condensa-
tion of 2,6 diformylpyridine and trans-1,2-diaminocyclohexane [15a] leading to a
3
3 macrocyclic Schiff base, which was then easily converted into the correspond-
ing macrocyclic amine. The chiral macrocycle
L
was obtained in the enantiopure
forms
L
RRRRRR
and
L
SSSSSS
, corresponding to an all-
R
or all-
S
configuration of the
diaminocyclohexane carbon atoms, respectively [18]. Mixing ligand
L
with Ln
3þ
precursors (Ln
þ
Eu,Tb,Yb)resultedintheformationofmetalcomplexes,asindi-
cated by
1
H-NMR spectroscopy, which were further isolated as enantiopure nitrate
salts. The X-ray crystal structure of (
M
)-[Ln
L
RRRRRR
]
3þ
complexes revealed that
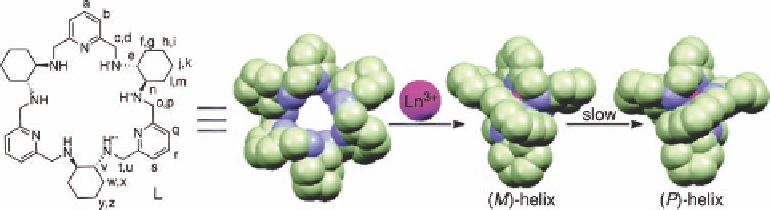
they all adopt a unique type of geometry. Because the cavity radius of the “open”
form of the ligand is too large to accommodate a single Ln ion, the macrocycle
wraps around the cation in a helical fashion, leading to the generation of a left-
handed M double helix.
The
1
H-NMR spectra of the (
M
)-[Ln
L
RRRRRR
] complexes reflect their relatively high
stability in solution. For instance, the
1
H-NMR spectrum of a water solution of
(
M
)-[Eu
L
RRRRRR
]
3þ
shows only traces of the (
P
) complex after three weeks. The
(
M
)-[Yb
L
RRRRRR
]
3þ
complex, however, is somewhat less stable because, in water, it
gradually converts into the (
P
) paramagnetic complex. After refluxing for 15 h, equili-
brium is reached, with 95% conversion into the (
P
)-[Yb
L
RRRRRR
]
3þ
complex. The pro-
cess can also be observed by CD spectroscopy, which reveals profound differences
between the two forms. The inversion in helicity is explained by the notion that the less
stable (
M
)-[Yb
L
RRRRRR
]
3þ
isomer is a kinetic product of the complexation of the free
¼
Figure 11.4 Schematic representation of macrocycle L (left), helix formation upon binding to
a lanthanide ion and helix inversion, as demonstrated by the groups of Muller and Lisowski.
Reprinted with permission from Ref. [17]. Copyright 2008 American Chemical Society.
Search WWH ::

Custom Search