Chemistry Reference
In-Depth Information
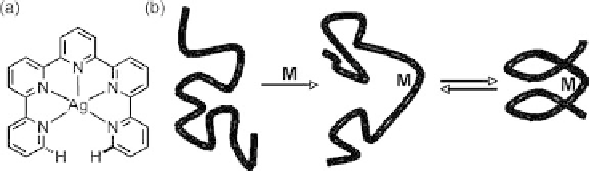
Figure 11.1 Classification of metallofoldamers: (a) templated helix-like structures and
(b) nucleated secondary structures [6].
The coordination of metal ions to single-stranded oligomers can either template a heli-
cal structure or nucleate its formation (Figure 11.1) [6]. J. Fox at the University of Dela-
ware defined molecules that template an abiotic helix, such as the one represented in
Figure 11.1a, as having an intrinsically helical metal coordination sphere. “Helicates” [7]
are one example of molecules that template an abiotic helix, and they have been broadly
discussed in earlier chapters. In contrast, molecules such as the one represented in
Figure 11.1b do not have an inherently chiral metal coordination sphere, but instead,
metal binding is likely to impose conformational restrictions which drive the folding of
the biomimetic oligomer chain into a stable three-dimensional structure. This nucleation
process resembles the folding of natural polypeptides and proteins in which metal coordi-
nation leads to a specific folding pathway by lowering the entropy of the unfolded state,
thus speeding up the folding event [8].
Some metallofodamers are generated as a combination between a templated helical or
other folded structure and a nucleated three-dimensional structure. In these cases, a metal
ion will template a specific folding event and the resulted fold will then enable the nuclea-
tion of a stable structure by additional non-covalent interactions. This chapter will discuss
such biomimetic metallofoldamers as well as abiotic single-stranded metallofoldamers,
which formed solely upon nucleation by metal ion coordination. The discussion will not
be limited to helical folding but rather will be further extended to metallofoldamers with
various three-dimensional architectures.
11.2 Single-Stranded Oligomers in Which Metal Coordination Templates,
or Templates and Nucleates the Formation of an Abiotic Helix
As shown in natural systems, an ordered structure within a biopolymer depends upon a
combination of different physicochemical interactions [4]. Based on this notion, scientists
began to develop abiotic oligomers in which metal coordination promotes various non-
local interactions, leading to a controlled folded structure. The first example of an abiotic
oligomer whose structure in solution was designed to involve both non-specific (solvo-
phobic [9]) and specific (metal coordination) interactions, was reported by the group of
Moore in 1999 [10]. The solution behavior of a meta-connected oligomer whose back-
bone consists of 12 non-polar phenylacetylene units was tested in the context of metal
Search WWH ::

Custom Search