Chemistry Reference
In-Depth Information
Figure 8.5
Schematic representation of a double- and a triple-stranded dinuclear helicate.
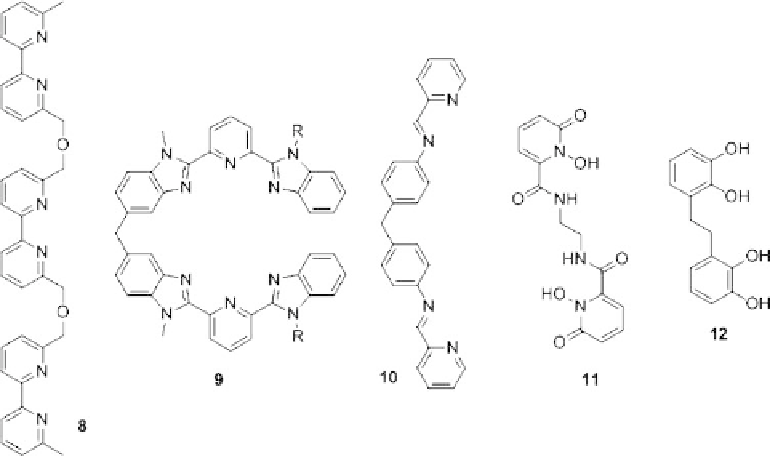
Figure 8.6 shows representative examples of oligodentate ligands used in helicate
chemistry [23-26].
Starting in the 1990s, catechol ligands like
12
were intensely studied. Upon deprotona-
tion of the catechol unit, an effective ligand system for the formation of helicates is
obtained.
12
and related derivatives were used to study the mechanisms of the self-assem-
bly, structure, ability to form host-guest complexes, and stereochemical features of the
oligonuclear helicates [27]. In the latter case it was found that appropriate design of the
spacer enables the diastereoselective formation of either the “classical” helicates or the
meso-form. In the meso-form one metal complex unit is configurated L and the other D.
Those non-chiral coordination compounds with two helical domains possessing opposite
configuration are termed side by side complexes, meso-helicates, or mesocates [28].
Today the chemistry of helicates still represents an evolving field of research, due to the
model character of the relatively simple metallosupramolecular species. Intense study of
Figure 8.6 Representative examples for ligands forming helicates in the presence of appro-
priate metal ions.

Search WWH ::

Custom Search