Chemistry Reference
In-Depth Information
the helicates allows a deeper insight into mechanisms which are also active for much
more complicated systems. In addition, “function” (e.g., metal-metal communication)
has become important. As a further special aspect of helicate chemistry the structural
organization of linear ligand strands upon metal coordination has to be mentioned.
8.3.2 Peptide-Helicates
In helicates, two or more metal ions are connected through oligodentate ligands bearing
connecting bridges. In this context, it became of interest whether simple amino acids or
short peptides can be used as spacers of the helicating ligands. Those would allow the
investigation of the mutual interplay of structural information at the metal and at the pep-
tide. The self-assembly of well defined complexes should be controlled by a subtle coop-
eration of the different effects.
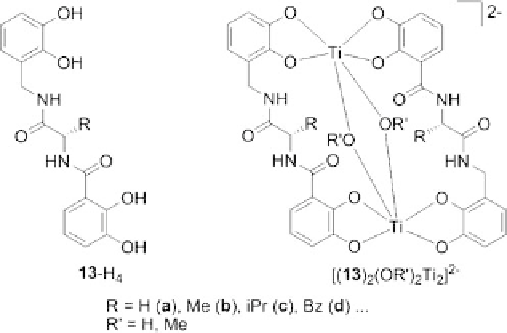
Thus, amino acid bridged dicatechol ligands
13
-H
4
were prepared by standard peptide
coupling reactions using common protecting group strategies (Figure 8.7) [29].
Complexation of the ligands
13
-H
4
with titanium(IV) ions under basic conditions did
not lead to the formation of triple-stranded helicates [30]. ESI MS showed that double-
stranded coordination compounds of the type [(
13
)
2
(OR
0
)
2
Ti
2
]
2
with hydroxyl or alco-
holate coligands are formed. However, mass spectrometry cannot distinguish between dif-
ferent isomers. Proton NMR, in contrast, shows a collection of several isomers to be
present. This is due to different relative orientations of the amino acid bridged ligands
(parallel or antiparallel) and to different configurations at the metal complex units (L or
D). In total, seven different isomers can be formed (Figure 8.8).
Figure 8.9 shows representative example proton NMR resonances of one of the diaster-
eomeric benzyl protons in the NMR spectrum of [(
13d
)
2
(OMe)
2
Ti
2
]
2
. Initially (15 h
after preparation of the sample), seven different species can be observed in the spectrum.
After 14 days several of the signals nearly disappeared and one species is highly domi-
nant. This means that initial complex formation proceeds unspecifically under kinetic
Figure 8.7 Amino acid bridged dicatechol ligands and their respective dinuclear double-
stranded titanium(IV) complexes (only the isomer with parallel orientation of the ligand
strands is shown).
Search WWH ::

Custom Search