Agriculture Reference
In-Depth Information
surface phenomena should, therefore, enable a viticulturalist to better manage vine-
yard soils.
As indicated in chapter 2, the crystalline clay minerals and sesquioxides have
electric charges that attract cations and anions from the surrounding soil solution.
Soil organic matter (
SOM
) also has a negative charge that varies with soil pH. The
permanent negative charge in the clay minerals, which have thin laminar struc-
tures (see box 2.4), acts as if it were spread over the planar surfaces of the crys-
tals. The hydrophilic carboxylic and phenolic groups in organic matter also point
into the soil solution. This negative charge attracts a surplus of cations (the
coun-
terions
) into the solution adjacent to the surface. At the same time, anions (the
co-ions
) are repelled to create a deficit of these ions in solution near the surface.
The combination of fixed surface charges and mobile ions of opposite charge in
solution comprises a
double layer
. Overall, the double layer is electrically neutral.
However, the electrostatic force between the fixed charges and mobile ions
in the double layer is diminished by the high dielectric constant of water. The at-
tractive (or repulsive) force acting on the mobile ions is also opposed by a diffu-
sive force pulling these ions away from a region of high concentration to one of
lower concentration. At equilibrium, the electrostatic force per unit area of sur-
face is just balanced by the difference in osmotic force per unit area between the
surface and the soil solution well removed from the surface. The result is a nonuni-
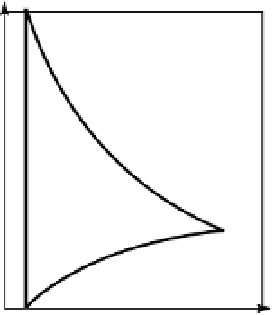
form distribution of cations and anions in solution, illustrated in figure 4.6a, in
which the ion concentrations change exponentially as the surface is approached
(fig. 4.6b). The combination of fixed surface charges and distributed mobile
charges is called a
diffuse double layer
(
DDL
). Such a
DDL
develops at surfaces
where the charge is permanent (constant), such as clay mineral planar surfaces, or
the charge is variable, depending on the solution pH, such as clay mineral edge
faces, oxide surfaces, and organic matter. Box 4.3 gives an example of how the
charge on a kaolinite edge face, or an oxide surface, changes with pH.
4.5.2
How the Diffuse Double Layer Can Change
The
DDL
at charged surfaces is dynamic. Its effective thickness is measured by
the average distance from the surface over which the concentration of the mobile
(a) The distribution of mobile cations (+) and anions (-) with distance from a negatively
charged surface. (b) Ion concentrations relative to the bulk solution concentration
C
o
(White 1997). Reproduced with permission of Blackwell Science Ltd.
Figure 4.6
Solution
+
+
+
+
+
+
-
-
+
+
+
+
+
Cation surplus
-
-
+
+
+
+
+
+
+
-
+
+
+
-
+
+
+
-
+
+
-
+
+
+
+
-
+
+
-
+
+
-
C
o
-
+
+
-
+
+
+
+
+
-
+
-
Anion deficit
+
+
+
+
+
+
+
(a)
+
(b)
Distance