Agriculture Reference
In-Depth Information
Leaf fall,
prunings
Compost
Organic P
Fertilizer P
(inorganic)
Mineralization,
immobilization
Soil solution
P
Uptake
Labile P
Fast
Mycorrhizae
(organic and
inorganic P)
Adsorbed P
Slow
P in insoluble compounds
}
Strongly sorbed P and
Nonlabile P
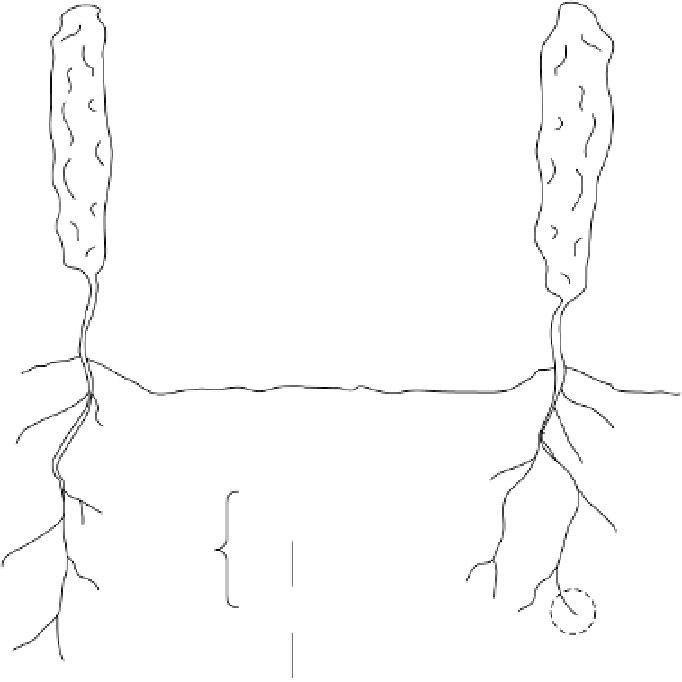
P cycle for a vineyard soil system.
Figure 4.5
the reasons discussed in section 4.5.4. This means that the P concentration in the
soil solution is normally very low (
0.01 mg/L). Sorbed ions that are easily de-
sorbed, plus those ions already in solution, collectively comprise the
labile pool
of
a nutrient. Those ions that are very strongly adsorbed or trapped in insoluble com-
pounds and recalcitrant organic forms comprise the
nonlabile pool
. Nutrients in
the labile pool are considered to be “plant-available.”
In addition to the adsorbed forms, P may exist in insoluble compounds with
Fe, Al, or Ca, especially around dissolving fertilizer granules where high P con-
centrations and low pH exist for a time (section 5.4.2.1). In soils of semiarid to
arid regions, sulfate can accumulate in the profile as gypsum (CaSO
4
.2H
2
O).
Phosphorus cycling in the soil-plant system and transformations between labile
and nonlabile forms of P in the soil are illustrated in figure 4.5.
Partitioning of Ions Between the Solid and Solution Phases
4.5
The Diffuse Double Layer
The way in which nutrient ions are retained, or released, and how the ionic com-
position can affect a soil's physical properties depend very much on the behavior
of cations and anions at organic and mineral surfaces. A basic understanding of
4.5.1