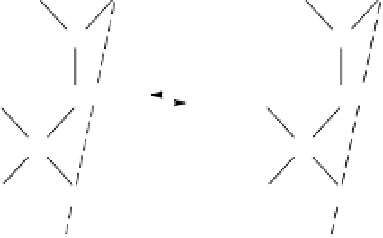Agriculture Reference
In-Depth Information
Box 4.3
pH-Dependent Charge on Mineral Surfaces
Oxygen and OH groups coordinated to Si and Al atoms in a kaolinite crystal
are exposed at the crystal edges, and so have potentially unneutralized charges.
These are satisfied by H
ions from solution, which become tightly bound to the
O and OH groups. The H
ions enter into the surface plane of atoms and change
the charge on that surface, as shown in figure B4.3.1. The surface groups behave
like weakly dissociating acids (see appendix 3), and a reversible equilibrium
involving H
ions is set up between the surface and solution. At low pH (high
H
ion activity), the surface acquires a net positive charge. As the pH rises (lower
H
ion activity), the tendency for H
ions to dissociate from the surface increases,
so that at high pH the surface charge becomes negative. At some intermediate pH,
the surface bears no net charge. This pH defines the
point of zero charge
(
PZC
).
Figure B4.3.1
pH-dependent charges at a kaolinite edge face (White 1997). Reproduced with permis-
sion of Blackwell Science Ltd.
Decreasing pH
O
OH
O
OH
O
OH
Si
Si
Si
H
+
H
+
1
2
1
2
1
2
OH
(+ )
OH
(+ )
O
(- )
Al
Al
Al
H
1
2
1
2
1
2
(- )
(- )
OH
O
(+ )
OH
OH
OH
OH
H
Edge charge +1
O
PZC 6-7
-1
(continued)
ions is affected by the fixed surface charges. The electrostatic force that attracts
mobile cations to a negatively charged surface, or repels anions, is directly pro-
portional to the charge on the ion. For cations, the force decreases in the order
Al
3
Ca
2
Mg
2
Na
K
. Thus, for a fixed concentration of salts in
the soil solution, we would expect a
DDL
made up of Al
3
ions to be much thin-
ner, or more compressed, than that made up of Na
ions.
In addition to the force pulling cations to the surface, we must consider the
countervailing osmotic force pulling them out into the bulk solution. Diffusion
outward depends on the difference in concentration for
individual
ion species be-
tween the surface region and the bulk solution. The concentration of whichever
cation is attracted to the clay surface (e.g., Na
) is determined by the charge on
the clay. Therefore, the difference in concentration across the
DDL
, and hence
the outward diffusive force, will change as the bulk solution concentration changes.