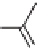Chemistry Reference
In-Depth Information
from 2-alkynoic acids and chiral 1,3-oxazolidin-2-ones,
24, 53
efficiently undergo
intermolecular PKRs with strained olefins (norbornene, norbornadiene) in good yields
and, in several instances, with excellent diastereoselectivities (up to 17.5:1 dr; see Table
5.8 for selected results with norbornadiene). The regioselectivity of the process is the
following: with the phenylpropiolate (
67
) or trimethylsilylpropiolate (
68
) derivatives, the
Tab l e 5 . 8
O
O
H
Xc
Co
2
(CO)
8,
rt;
O
R
+
R
O
Xc
H
Xc
Me
67a,b
(R = Ph)
68a
(R = SiMe
3
)
69a,b
(R = Me)
O
To l u e n e ,
rt-60 °C,
or NMO, CH
2
Cl
2
(for
68a
)
71a,b
70a
-
d
(Major diast.)
Alkyne
Yield (%)
R
Xc-
Product
Dr
O
67a
Ph
70a
96
5.2:1
ON
Ph
O
ON
SiMe
3
70b
88
3.6:1
68a
Ph
O
70c
53
7.6:1
ON
69a
Me
71a
23
1.9:1
Ph
Me
Me
O
O
97
14:1
70d
67b
Ph
N
H
Me
H
Me
Me
70e
34
17.5:1
O
O
69b
Me
N
71b
38
5.1:1
H
Me
H