Chemistry Reference
In-Depth Information
12.9
High purity water
12.9.1
Chloride, sulphate, nitrite
Roberts
et al.
[80] have described a single column ion chromatographic method for the
determination of chloride and sulphate in steam condensate and boiler feed water. This
was shown to be a valuable technique for analysing µg L
−1
levels of chloride and
sulphate in very pure waters. The anions are concentrated on a short precolumn,
separated on a low capacity ion exchange column and detected by an electrical
conductivity detector. The apparatus is simple and no 'suppressor
'
column is needed.
Adaptation to on-line analysis would be inexpensive and automation would require
control of only the load/ inject valve.
The preliminary work by Roberts
et al.
[80] was carried out using a resin of very low
exchange capacity (0.003mequiv g
−1
) and a 7.5×10
−4
M solution of benzoic acid as the
eluant. Unusually good sensitivity can be obtained in single column anion
chromatography using this eluant. To increase the sensitivity further, the size of the
sample loop was increased to 500µL from the usual 100µL. This method adequately
separated chloride and sulphate with detection limits of 10mg L
−1
chloride and 100mg
L
−1
sulphate. However, a prolonged dip in the base line occurred sometime after the
sulphate peak and made this procedure inefficient for repetitive analyses. Chloride must
be separated from the water dip before it can be analysed reliably in the same run with a
sulphate. This was accomplished by carefully constructing a concentrator column and by
careful choice of the column dimensions, resin capacity and eluant strength. The effect of
column and eluant parameters on anion retention times has been discussed previously by
Gjerde
et al.
[81,82]. If eluant flow rate and anion selectivity coefficients are taken to be
constant then the effects of column resin weight
w,
resin capacity
ic,
and eluant
concentration
[E]
on the adjusted retention time of anions,
t',
are shown by the following
equation where
y
is the sample anion charge and
x
is the eluant anion charge.
It can be seen that the log of column resin weight can be directly proportional to log
adjusted retention time regardless of the anionic charges. Increasing the weight of resin in
a column will shift both chloride and sulphate to later retention times. However, lowering
the resin capacity and/or increasing the eluant concentration will shift the sulphate faster
relative to chloride to shorter retention times. These parameters were adjusted until
chloride eluted away from the water dip and sulphate still eluted in a reasonable amount
of time. The determination of chloride and sulphate in one run greatly reduced the time
needed to do the analysis. Sample concentrations for which peaks were at least three
times the background signal were 3-5µg L
−1
chloride and 1-2µg L
−1
sulphate with this
procedure.
A chromatogram of the determination of chloride and sulphate in a single run is shown
in Fig. 12.39. Data from 20ml injections of 5-100µg L
−1

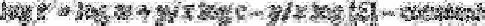
Search WWH ::

Custom Search