Chemistry Reference
In-Depth Information
retained on the column and slowly dissociate during the
Table 12.32
Percentage of total cyanide in metal complexes determined as 'free' cyanide
log β
t
Metal complex
%
Cd(CN)
4
2-
18.8
102
Zn(CN)
4
2−
16.7
102
Ni(CN)
4
2−
31.3
81
Cu(CN)
4
3-
30.3
52
Cu(CN)
3
2−
28.6
42
Cu(CN)
2
−
24.0
38
Au(CN)
2
−
38.3
0
Fe(CN)
6
3-
42
0
Co(CN)
4
3−
64
0
Source: Reproduced with permission from the American Chemical Society [23]
chromatography. This slow dissociation produces tailing which lasts for several minutes
as the free cyanide elutes and is detected. As the results presented in Table 12.32
demonstrate, the tailing and the non-quantitative recovery of cyanide preclude the use of
direct injection to determine total cyanide in samples containing copper and nickel. These
samples may be analysed after acid distillation and caustic trapping. The cyanide in the
caustic solution can then be determined by ion chromatography with electrochemical
detection.
Category 3 includes those cyanides which are inert and therefore totally undissociated,
such as 42) and
No free cyanide was detected for these complexes. Although
these complexes do not elute under the chromatographic conditions used, they can be
eluted and determined by using different chromatographic conditions and conductivity
detection.
Samples containing both free cyanide (or weakly complexed strongly complexed
cyanide can be analysed for free cyanide by direct injection. The determination of total
cyanide (both free and strongly complexed) requires distillation of the sample with
caustic trapping.
12.8.4
Sulphite and dithionate
Petrie
et al.
[53] have determined sulphite and dithionate in mineral leachates by ion
chromatography.

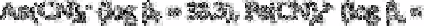


Search WWH ::

Custom Search