Chemistry Reference
In-Depth Information
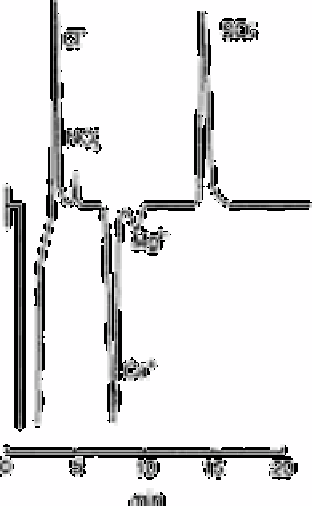
Fig. 12.33
Chromatogram of water
Source: Reproduced with permission from the American Chemical
Society [2]
value of noise of the base line were 0.04mg L
−1
for chloride 0.05mg L
−1
for nitrite,
nitrate, magnesium and calcium and 0.1mg L
−1
for phosphate and sulphate.
The retention times observed for calcium and magnesium injected as metal cations and
those injected as EDTA chelate anions were not significantly different. Therefore, an
alkaline earth metal (M
2+
) forms a chelate anion (M(EDTA)
2−
) immediately upon contact
with EDTA eluent and is then separated according to the following exchange reactions.
In this case, the H
2
EDTA
2−
and HEDTA
3−
anions were assumed to present nearly
equimolar concentrations in the eluent at pH 6.0:
Using the EDTA eluent, magnesium and calcium chelate anions were eluted between
nitrate and sulphate as shown in Table 12.20 and also in Fig. 12.33. Negative peaks of
chelate anions might be caused by the lower conductivity of the chelate anion relative to
that of the EDTA eluent anion.
The analytical results are given in Table 12.21.


Search WWH ::

Custom Search