Chemistry Reference
In-Depth Information
Table 12.21
Analytical results on rain water
mg L
−1
Cl
−
NO
3
−
SO
4
2−
Ca
2+
Mg
2+
2.5
1.1
0.38
0.9
0.5
Source: Reproduced with permission from the American Chemical Society [2]
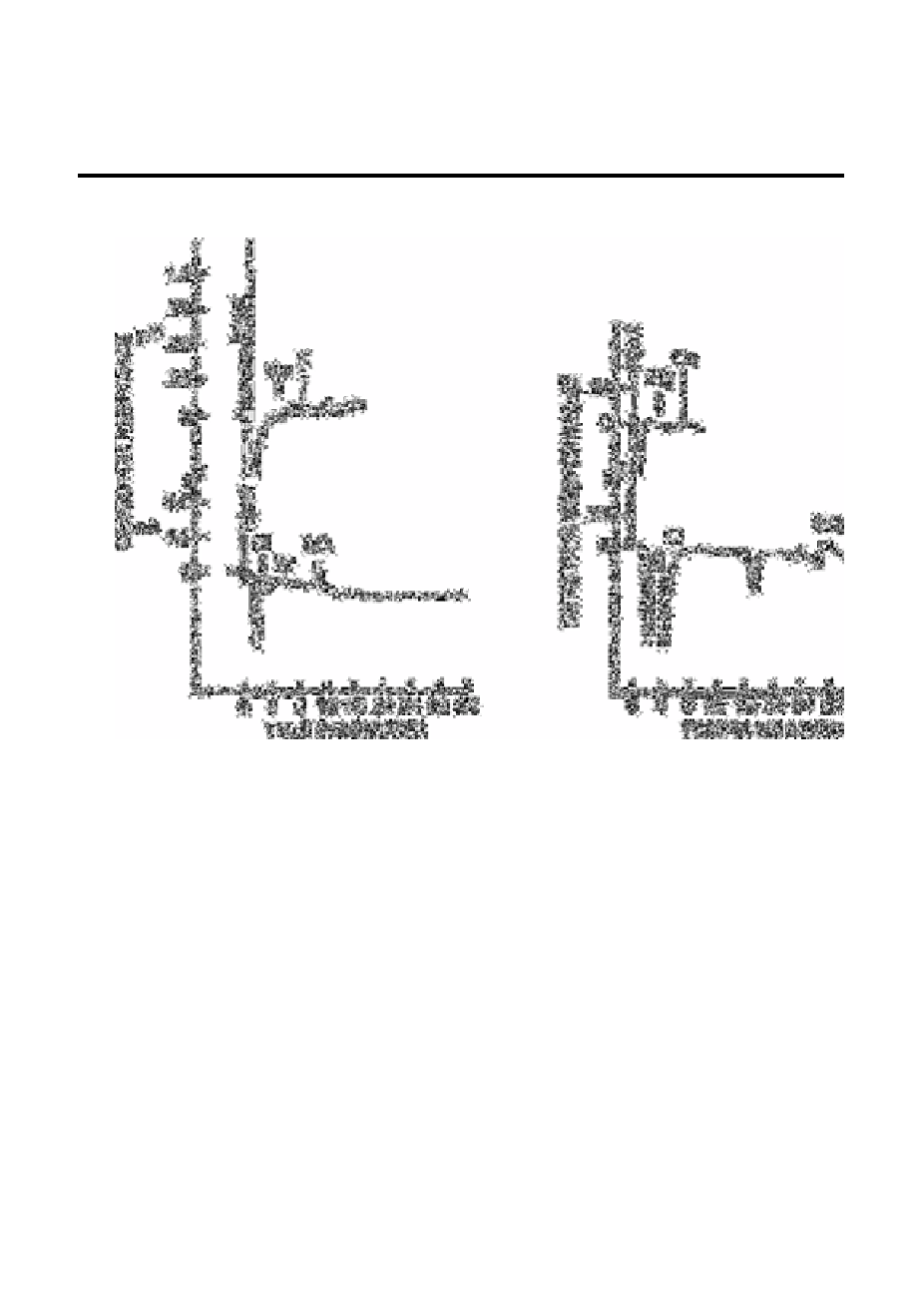
Fig. 12.34
Chromatograms of rainwater (Fort Worth, Texas) monovalent
cations and anions and divalent cations and anions
Source: Reproduced with permission from the American Chemical
Society [63]
Ion chromatographic methods have been described for the co-determination of anions and
cations in rainwater. Thus Jones and Tarter [63], using the conditions given in Table
12.22 reported determinations down to 1mg L
−1
of anions (chloride, bromide and
sulphate) and cations (sodium, potassium, magnesium and calcium) in rainwater (Fig.
12.34) without converting the cations to anion complexes prior to detection [64]. The
technique uses a cation separator column, a conductivity detector, an anion suppressor
column, and either a second conductivity detector or an electrochemical detector in
sequence. The use of different eluants provides a means for the detection of monovalent
cations and anions and divalent cations and anions in each of the samples. Using an
eluant with
Search WWH ::

Custom Search