Chemistry Reference
In-Depth Information
This is avoided by working at 70°C, where equilibrium is obtained in less than 20min. An
essential factor is the purity of the acid utilised because if the sulphuric acid has only
average purity, an increase in the photodecomposition of the blanks is observed and the
spectral characteristics of the complex are modified.
Kristalev and Shevchenko [84] carried out a comparative study of methods for the
determination of boron with 2,4-dihydroxybenzo-phenone and resacetophenone. They
deduced that the former method is more sensitive but the fluorescence development is
slow, the intensity depending on the ultraviolet irradiation time and the intensity
decreasing in the presence of phosphate. The second complex forms almost
instantaneously and is resistant to ultraviolet irradiation.
Marcantonatos
et al.
[85] studied the reactions of boric acid with nine substituted 2-
hydroxybenzophenones (see below (iv)) in concentrated sulphuric acid: 2-hydroxy-4-
methoxy-4
′
-chlorobenzophenone, 2hydroxy-4-methoxy-4
′
-methylbenzophenone,
2,2
′
,4,4
′
-tetrahydroxybenzophenone, 2,2
′
-dihydroxy-4,4
′
-dimenthoxybenzophenone, 2,2
′
dihydroxy-4-methoxybenzophenone, 2,2
′
-dihydroxy-4-n-octyloxybenzophenone, 2-
hydroxy-5-chlorobenzophenone, 2-hydroxy-5-methylbenzophenone and 2,4,4
′
-
trihydroxybenzophenone. All of these reagents form fluorescent complexes with boric
acid. Their excitation spectra indicate that these complexes are similar in structure, and
the fluorescence spectra show that the composition and intensity of fluorescent light
depend on the electronic nature of the various substituents of the complexing agents. All
of these reagents can be used for the determination of submicrogram amounts of boron,
but 2-hydroxy-4-methoxy-4
′
-chlorobenzophenone is the most sensitive.
(iv)
These workers also thoroughly studied the influence of some properties of the medium on
the fluorescence of the boric acid—2-hydroxy-4methoxy-4
′
-chlorobenzophenone
complex [86]. When concentrated sulphuric acid is replaced by glacial acetic—
concentrated sulphuric acid, the intensity of fluorescence increases considerably. The
method has been applied to the determination of trace amounts of boron in
analyticalreagent grade sodium hydroxide [86], steels [87], plants [88], waters [89] and
blood [44, 90]. Afghan
et al.
[91] developed an automated method based on this reaction
for the determination of boron in non saline waters.
Other reagents
Shcherbov and Korzheva [92, 93] suggested a method for the determination of boron
with phenylfluorone (see below (v)) in aqueous alkaline medium where the complex
formed has a dark blue fluorescence [94]. On standing, the fluorescence intensity in
solutions containing the reagent changes; in the blank solution it decreases, but in the
solution containing boron it becomes higher; after a 24h period, 1µg of boron can

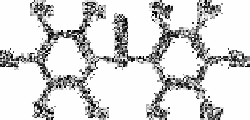

Search WWH ::

Custom Search