Chemistry Reference
In-Depth Information
fluorometers using the reaction of boron with benzoin. They showed that oxygen should
be removed from this system and, although the maximum excitation is at 365nm, they
recommended 405nm in order to decrease the decomposition of the reagent. In a later
paper [46], these authors applied the method to the determination of boron in silicon
(limit of detection about 0.03ppm) in seawater (with a precision of better than 2%).
White and Hoffman [74] showed that it is desirable to use a glycine buffer of pH 12.8
because the intensity readings are more stable than those obtained when using the
original procedure in which sodium hydroxide was used. Elliot and Radley [76] also
studied the deactivating effects of oxygen in this system and investigated other solvents
in the hope of increasing the sensitivity of the reaction. They showed that the intensity of
fluorescence when formamide is used is stronger than that when ethanol is used. They
discussed the possible relationship between the dielectric constant of the solvent and the
fluorescence intensity of the system. Other workers have applied this method to the
determination of boron in raw material with iron [77] and in soils [78].
Boric acid forms a highly sensitive luminescent complex with dibenzoylmethane (ii) in
concentrated sulphuric acid [51,79], which is proposed for the trace determination of
boric acid. The limit of detection is 0.5ng mL
−1
.
(ii)
Neelakantam and Rao [80] proposed the determination of boric acid with
resacetophenone (see below (iii)). In concentrated sulphuric acid a brilliant blue
fluorescence is obtained with trace amounts of boric acid under ultraviolet radiation. A
disadvantage of the method is the necessity of working under filtered ultraviolet
radiation. In a later paper [81], various substituted resacetophenones were investigated in
an attempt to find a material that would emit fluorescence in the visible range. No shift
from the ultraviolet to the visible region were found, i.e. an increase in relative molecular
mass did not bring about any shift of the fluorescence into the visible region. In the same
paper, methods of overcoming interferences were discussed. The replacement of
concentrated sulphuric acid by syrupy phosphoric acid rendered the method more
sensitive [82], and also obviated the interference of some ions such as bromide, iodide
and nitrate.
(iii)
2,4-dihydroxybenzophenone forms a fluorescent complex with boric acid in sulphuric
acid medium [83], with a stoicheiometry of 1:1. Maximum fluorescence intensity is
obtained after 10h. Under these conditions the blanks give a relatively high fluorescence
because the reagent suffers partial oxidation and is converted into fluorescent products.

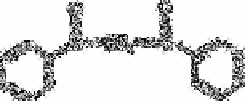

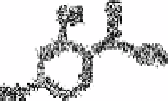
Search WWH ::

Custom Search