Biology Reference
In-Depth Information
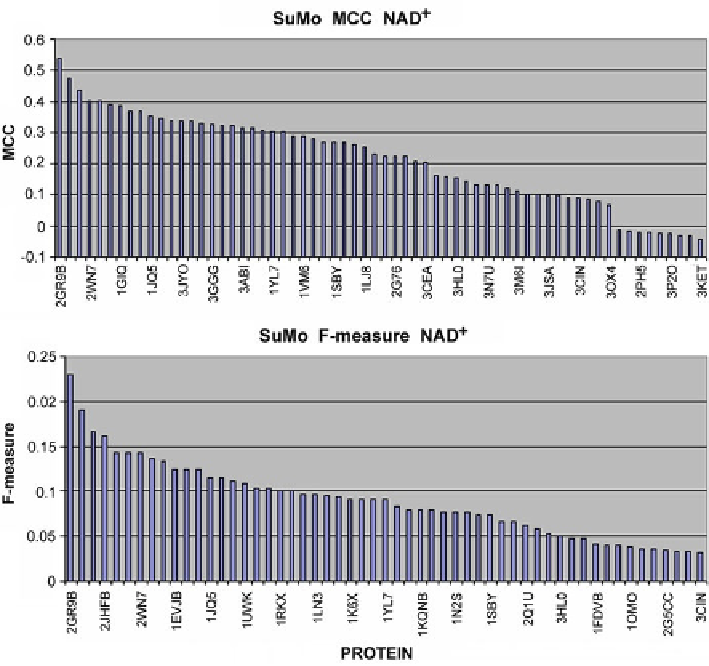
Fig. 4.6
Comparison of MCC and F-measure results for NAD
+
-complexing proteins as reported
by Sumo
We can conclude that the pocket to which this ligand binds (and hence the biological
function performed by the protein) remain fairly conservative from an evolutionary
viewpoint.
The FOD model yielded significantly lower scores for FMN binding sites com-
pared with NAD
+
(Fig.
4.11
). Of particular note are the low values of the F-measure
metric. This suggests that the mechanism responsible for FMN complexation affects
the structure of the protein's hydrophobic core to a far lesser degree than in the case
of NAD
+
. Indeed, FMN complexation is relatively static, i.e. the ligand remains
securely lodged in a specific binding pocket, whereas NAD
+
binding is more
dynamic and the complex exists only for a brief while, limiting the likelihood of
errors. This is why it may be easier to distinguish NAD
+
complexation sites.
Similarly to other tools (except the FOD model), SuMo reports higher values of
MCC and F-measure metrics for FMN as compared to NAD
+
(Fig.
4.12
). It appears
that FMN binding sites are more accurately determined by the geometry of the binding
pocket than NAD
+
binding sites. MCC scores seem fairly consistent, while F-measure
values are more varied.
Search WWH ::

Custom Search